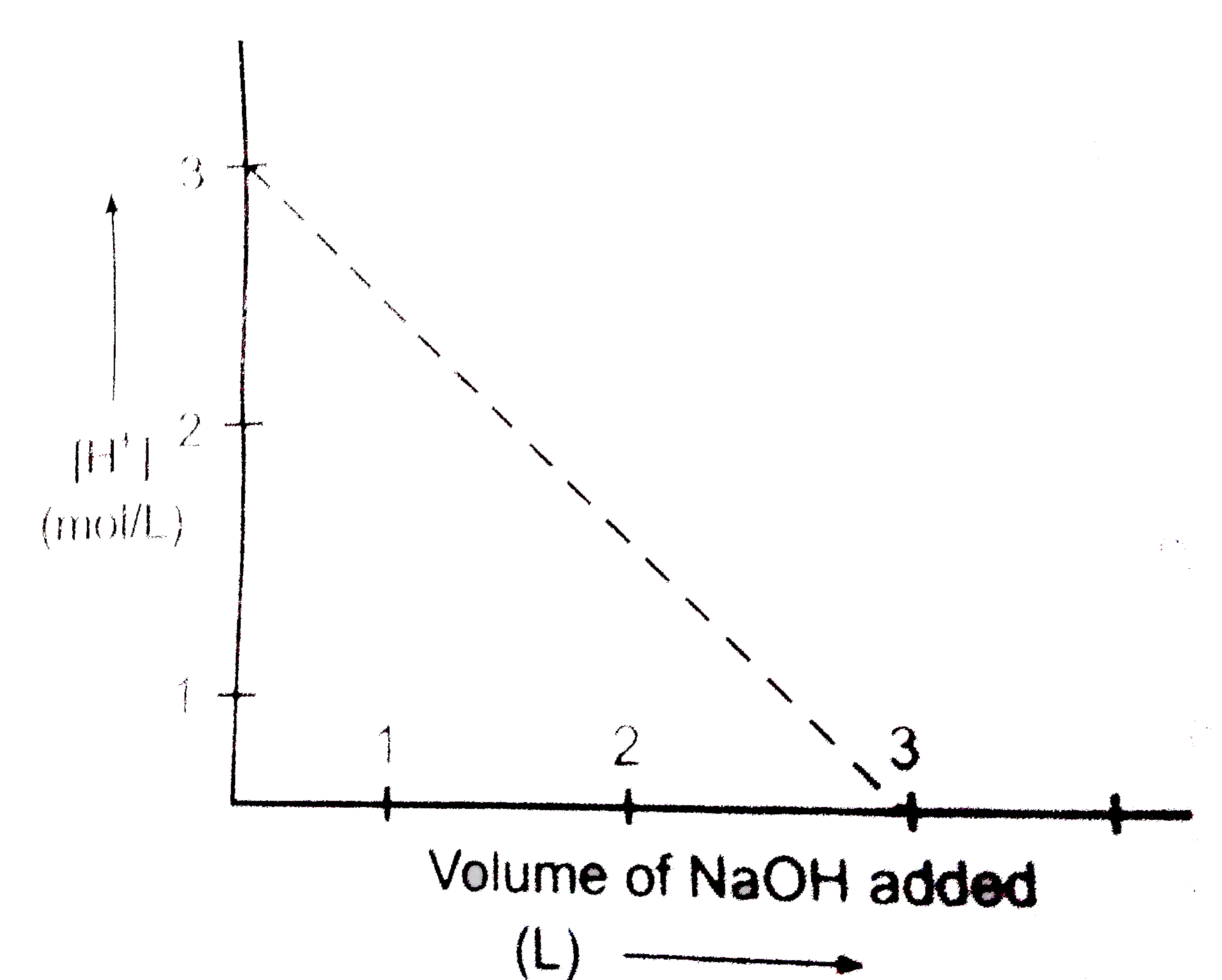
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 2 (Q.31 To Q.35)|5 VideosSTOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 3 - Passage|18 VideosSTOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 1 (Q.181 To Q.200)|20 VideosSOLID STATE
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|2 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-STOICHIOMETRY-Level 2 (Q.1 To Q.30)
- 6.2 g of a sample containing NaHCO(3), NaHCO(3) and non -volatiale in...
Text Solution
|
- Nitric acid can be produced from NH(3) in three step process I) 4NH(...
Text Solution
|
- 1 M NaOH solution was slowly added in to 1000 mL of 183.75 g impure H(...
Text Solution
|
- Inniting MnO(2) in air converts it quantitctively to Mn(3)O(4). A samp...
Text Solution
|
- A 1.0g sample of a pure organic compound cotaining chlorine is fused w...
Text Solution
|
- A 0.6gm sample consisting of only CaC(2)O(4) and MgC(2)O(4) is heated ...
Text Solution
|
- A metal M forms the sulphate M(2)(SO(4))(3). A 0.596 gram sample of t...
Text Solution
|
- Urea(H(2)NCONH(2)) is manufactured by passing CO(2)(g) through ammonia...
Text Solution
|
- 11.6 g of an organic compound having formula (C(n)H(2n+2)) is burnt in...
Text Solution
|
- H2O2 + 2KI overset("40% yield")to I2 + 2KOH H2O2 + 2KMnO4 + 3H2SO...
Text Solution
|
- SO(2)Cl(2) (sulphuryl chloride ) reacts with water to given a mixture ...
Text Solution
|
- 5 g sample contain only Na(2)CO(3) and Na(2)SO(4) . This sample is dis...
Text Solution
|
- 20 mL of 0.2 M NaOH(aq) solution is mixed with 35 mL of this 0.1 ML N...
Text Solution
|
- A silver coin weighing 11.34 g was dissolved in nitric acid When sodiu...
Text Solution
|
- Two elements X (at.mass 16) ard Y (at. mass 14) combine to form compou...
Text Solution
|
- The conversion of oxygen to ozone occurs to the extent of 15% only. Th...
Text Solution
|
- RH(2) (ion exchange resin) can replace Ca^(2+) ions in hard water as R...
Text Solution
|
- 100 cm^(3) of a solution of an acid (Molar mass =98) containing 29.4 g...
Text Solution
|
- 20 mL of 0.1 M solution of compound NaCO(3).NaHCO(3).2H(2)O is titrate...
Text Solution
|
- A sample containing HAsO(2) (mol. Mass=108) and weighing 3.78 g is dis...
Text Solution
|