Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.31 To Q.60)|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.121 To Q.150)|1 VideosELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI|Exercise Assertin-Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
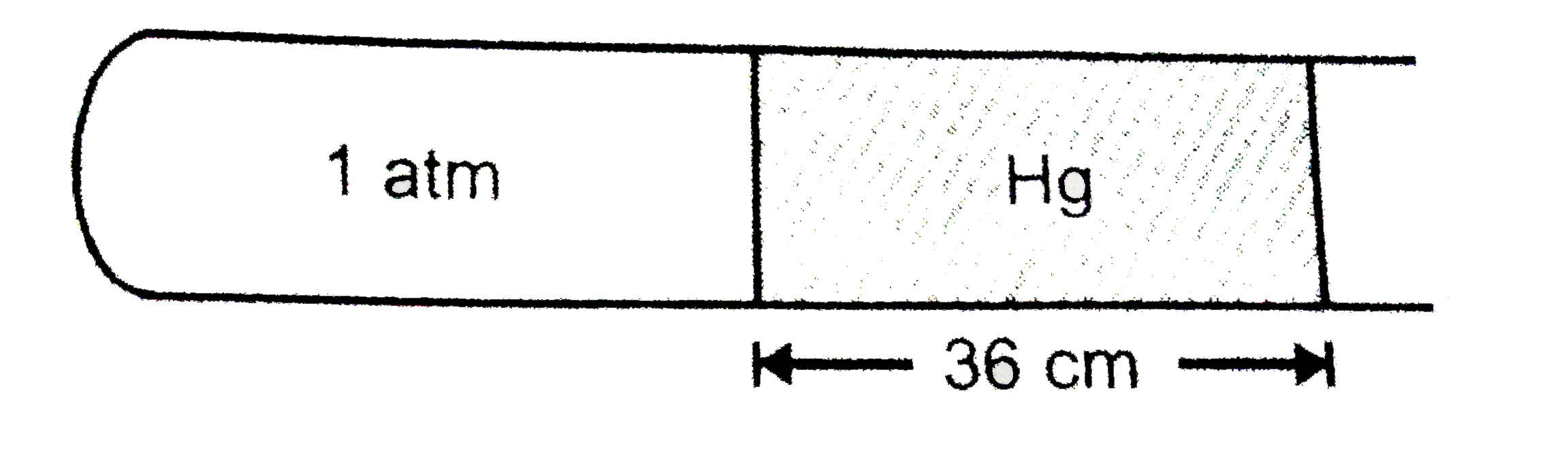 If the tube is held vertical keeping the open end up, lengh of air column shrink to 19 cm. What is the lengh (in cm) by which the mercury column shifts down?
If the tube is held vertical keeping the open end up, lengh of air column shrink to 19 cm. What is the lengh (in cm) by which the mercury column shifts down?