A. Water which gives ready and permanent lather with soap is called soft water. Water which do not give ready.and permanent lather with soap is called hard water.
Hardness of water is due to the presence of soluble chloride, sulphate and bicarbonate compounds of magnesium and calcium such as `MgCl_(2), MgSO_(4), Mg(HCO_(3))_(2), CaCl_(2), CaSO_(4), Ca(HCO_(3))_(2)`
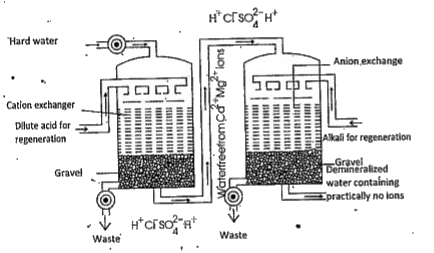
I) Ion exchange method : The water which is free from all the dissolved mineral salts is called deionised water.
Deionised water can be prepared in two steps.
i) Removal of cations : Hard water is passed through a tank containing cation exchange resin. Then the `Ca^(+2)` and `Mg^(+2)` ions present in the water are replaced by `H^+` ions from the resin.
`2R COOH + Ca^(+2) to (R(COO))_(2)Ca + 2H^(+))`
ii) Removal of anions : Now the water coming from the cation tank is passed througli a tank containing anion exchange resin. This resin absorbs anions like `Cl^(-), SO_(4)^(2-)` etc. from.the hard water and `OH^(-)` ions are released.
`RNH_(3)^(+) OH^(-) + Cl^(-) to RNH_(3)^(+) Cl^(-) + OH^(-)`
`2R NH_(3)^(+) OH^(-) + SO_(4)^(2-) to (R-NH_(3)^(+))_(2) SO_(4)^(-2) + 2OH^(-)`
Purification of water by ion exchange resins
The `H^+` and `OH^(-)` ions unite to form Deionised water.
After sometime the cation resin and the anion.resin lose their capacities to remove the cations and anjons from the hard water. Then the resins are said to be exhausted.- Then they are to be regenerated. The cation resin is regenerated by passing a solution of `H_2SO_4`.
Anion resin is regenerated by passing a moderately .concentrated solution of NaOH.
2) Calgon method : Sodium hexametaphosphate is commer-. daily called as calgon. When calgon is added to hard water it reacts with calcium.and magnesium ions forming complex an- `Na_(2)[Na_(4)(PO_(3))_(6)] + 2Mg^(2+) to Na_(2) [Mg_(2) (PO_(3))_(6)] + 4Na^(+)`
`Na_(2) [Na_(4)(PO_(3))_(6)] + 2Ca^(2+) to Na_(2) [Ca_(2)(PO_(3))_(6)] + 4Na^(+)`
Due to the formation of the complex the `Mg^(2+)` and `Ca^(2+)` become in active and cannot react with .soap. So the water can give good lather. This method is used only for laundry process.
