Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 10 -SECTION-B
- Deduce (a) Graham's law and (b) Daltons law from Kinetic gas equation.
Text Solution
|
- Balance the following equation in acid medium by Ion-electron method :...
Text Solution
|
- State and explain the Hess's law of constant heat summation.
Text Solution
|
- Derive the relation between K(p) and K(c) for the equilibrium reaction...
Text Solution
|
- What causes the temporary and permanent hardness of water?
Text Solution
|
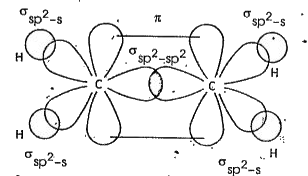
- Define sp^(2) Hybridisation. Explain the structure of Ethylene (C(2)H(...
Text Solution
|
- State Fajan's rules, and give suitable examples.
Text Solution
|
- Explain the structure of diborane.
Text Solution
|