A Preparation of Benzene:
1) Laboratory Method : Sodium benzoate on distillation with soda lime gives benzene.
`underset("Sod. Benzoate")(C_(6)H_(5)COONa + NaOH overset(CaO)underset(Delta)to C_(6)H_(6) + Na_(2)CO_(3))`
A mixture of NaOH and CaO is called soda lime.
(b) Polymerisation of acetylene : When acetylene gas is passed through red hot.copper tube, it undergoes polymerisation forming benzene.
`underset("Acetylene")(3C_(2)H_(2)) overset("Red hot Cu")underset(600^(@) C) to underset("Benzene")(C_(6)H_(6))`
Reason for not behaving as an alkene-.: Benzene has a number of resonance structures.
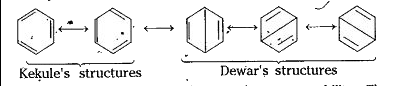
Because of resonance, benzene has more stability. The resonance energy.of benzene is also very high. It also indicates more stability to the benzene molecule. Hence, stability of a molecule is dueto saturation. Hence, from these two points it is evident that benzene exhibits more the properties of a saturated compound (alkane) rather than the properties of an , unsaturated compound (alkene). Methyl benzene from benzene : In presence of anhydrous. `AlCl_(3)`, benzene reacts with methyl chloride and forms methyl .benzene (Toluene).
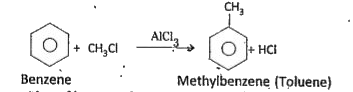
Preparation of benzene from acetylene: When acetylene gas is passed through red hot copper tube, it undergoes polymerisation forming benzene.
`underset("Acetylene")(3C_(2)H_(2)) overset("Red hot")underset("Cu tube") to underset("Benzene")(C_(6)H_(6))`
Benzene reactions
a) Halogenation : In presence of anhydrous `AlCl_(3)`, benzene reacts with `Cl_2` and gives chloro- benzene.
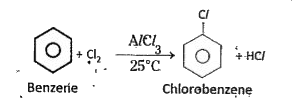
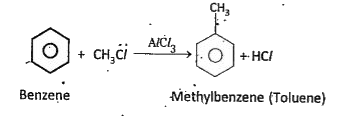
b)Alkylation: In presence of `AlCl_(3)` benzene reacts with alkyl, halides and gives alkyl benzene. This is known as Friedel Craft.s alkylation.
c) Acylation tin presence of `AlCl_(3)`, benzene reacts with acetyl chloride and.gives acetophenone.
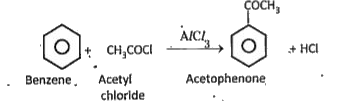
d). Nitration : Benzene reacts with nitration mixture below 60°C and gives -nitro- benzene.
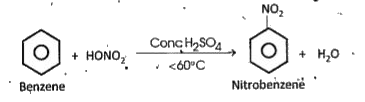
A mixture of conc. `H_(2)SO_(4)` and conc. `HNO_(3)` in `1:1` ratio by volume) is called nitration mixture.