Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 54-CHEMISTRY
- Bottels containing C(6)H(5) and C(6)H(5)CH(2)I lost their original lab...
Text Solution
|
- Phosphine, Acetylene and Amonia can be formed by treating water with:
Text Solution
|
- Which of the following represents the correct IUPAC name of the follow...
Text Solution
|
- A will be:
Text Solution
|
- Consider the compound given below. Select the correct statement a...
Text Solution
|
- For daniel cell reaction K(c)=10^(12) then E("cell")^(@) and the cell ...
Text Solution
|
- In the electrolysis of CuCl(2), solution, the mass of cathode increase...
Text Solution
|
- Radius of B^(-) in solid AB is 100 pm. If coordination number of A is ...
Text Solution
|
- Which curve is correct for zero order reaction?
Text Solution
|
- Latent heat of vaporisation of a liquid at 500 K and 1 atm pressure i...
Text Solution
|
- How many of the following ligands are chelating ligands? DMG,H(2)O,C(...
Text Solution
|
- Calculate the bond order of C-O bond in CO(3)^(2-) ion.
Text Solution
|
- The count of group 16 elment(s) that exists in theform of diatomic mol...
Text Solution
|
- How many primary amine functional group(s) is/are present in the foll...
Text Solution
|
- How many of the following alcohols are secondary in nature? Butan -2-o...
Text Solution
|
- (i) Find K(p) partial pressures of the component species and the volum...
Text Solution
|
- Find the vapour pressure of solution where 0.5 g pf a nonk volatile so...
Text Solution
|
- In a mixture containing 8.2 g Ca, 10 mm NaNO(3) , calculate the total ...
Text Solution
|
- A thin tube of uniform cross section is sealed at both ends. It lies h...
Text Solution
|
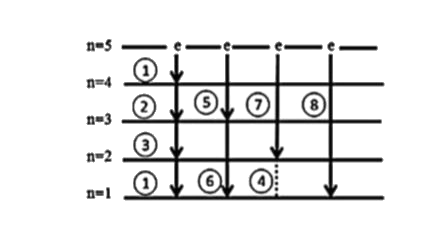
- Calculate the maximum no. of spectral lines obtained if 4-H-atoms are ...
Text Solution
|