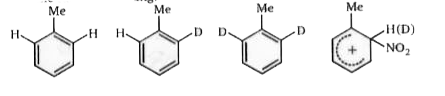
The rate determining step is the formation of the o-complex (with the nitronium ion). Hence, there will not be a kinetic isotope effect, since C -H or C - D bond fission is not involved in the rate determining step. The methyl group exerts a steric effect, but this will be the same for all three substrates (the attacking species is the same for all three). Therefore, the rates of nitration of all three will be the same.