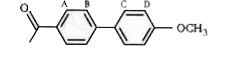
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MS CHOUHAN-AROMATIC COMPOUNDS -LEVEL-2 (SUBJECTIVE PROBLEMS)
- Which position will be attacked most rapidly by the nitronium ion (-NO...
Text Solution
|
- Double bond equivalent of D is :
Text Solution
|
- How many isomers 'X' of C(8)H(10) given only aromatic dicarboxylic aci...
Text Solution
|
- How many groups are op director in the electrophilic aromatic substitu...
Text Solution
|