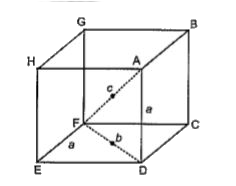
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE SOLID STATE
ICSE|Exercise MULTIPLE CHOICE QUESTIONS (Structure based questions)|6 VideosTHE SOLID STATE
ICSE|Exercise MULTIPLE CHOICE QUESTIONS (Assertion-Reason based questions)|10 VideosTHE SOLID STATE
ICSE|Exercise MULTIPLE CHOICE QUESTIONS (Match the following)|1 VideosSURFACE CHEMISTRY
ICSE|Exercise ISC EXAMINATION QUESTIONS |4 Videos
Similar Questions
Explore conceptually related problems
ICSE-THE SOLID STATE-MULTIPLE CHOICE QUESTIONS (Numerical based questions)
- A compound AB has a simple cubic structure and has molecular mass 99. ...
Text Solution
|
- An element has atomic weight 93 g "mol"^(-1) and density 11.5 g cm^(-...
Text Solution
|
- An element with density 2.8 g cm^(-3) forms a fee unit cell with edge...
Text Solution
|
- An element occurs in bcc structure. It has a cell edge length of 250 p...
Text Solution
|
- A bcc element (atomic mass 65) has a cell edge of 420 pm. Calculate it...
Text Solution
|
- Aluminium crystallises in a cubic close-packed structure. Radius of th...
Text Solution
|
- Aluminium crystallises in a cubic close-packed structure. Radius of th...
Text Solution
|
- An element A crystallizes in foc structure, 208 g of it has 4.2832 xx ...
Text Solution
|
- Calculate the efficiency of packing in case of metal crystal for simpl...
Text Solution
|
- An element crystallizes in a structure having fee unit cell of an edge...
Text Solution
|
- Calculate the packing efficiency of bcc structure.
Text Solution
|
- Gold has cubic crystal whose unit cell has an edge length of 407.9 pm....
Text Solution
|
- Gold has cubic crystal whose unit cell has an edge length of 407.9 pm....
Text Solution
|
- A metal has face centred cubic lattice. The edge length of the unit ce...
Text Solution
|