Ms Ruhi, a science teacher arranged the given apparatus where each beaker contains two different metal strips of same size, fastened together and immersed in hydrochloric acid.
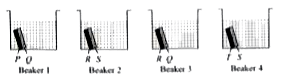
After 15 minutes, it was observed that the amount of Q ions formed is greater than P ions in beaker 1, S ions fonned is greater than R ions in beaker 2, R ions fonned is greater than Q ions in beaker 3 and T ions formed is greater than S ions in beaker 4.
On the basis of this observation, it can be concluded that the most reactive and the least reactive metals are respectively