The given figure shows the set-up to study the reaction between gas X and copper (II) oxide :
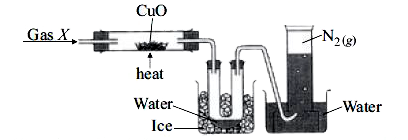
Which of the following statements are correct?
[Given : Atomic mass of N = 14 u, H = 1 , O = 16 u, C = 12 u]
I. Gas X is a compound of two elements, nitrogen and hydrogen.
II. The number of atoms present in 11.2 L of `N_(2)` is `6.023 xx10^(23)`.
III. 1 mole of `H_(2)O` contains 1 mole of oxygen molecules and 2 moles of hydrogen atoms.
IV. `N_(2)` gas being soluble in water is collected by upward displacement of water.