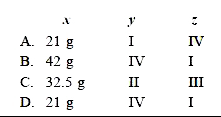Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -NSO QUESTION PAPER 2018 SET B -SCIENCE
- Four different experiments were conducted in the following ways: I. ...
Text Solution
|
- Read the given statements and select the correct option. Statement ...
Text Solution
|
- Following statements are given by four students Ajeet, Parth, Arunav a...
Text Solution
|
- Protein extract from a food sample was digested into amino acids and i...
Text Solution
|
- Tanvi was given three unknown metals P, Q and R. She performed the fol...
Text Solution
|
- Categorise the given atoms into groups of isobars and isotopes.
Text Solution
|
- A brief information about four substances P, Q, R and S is given. P...
Text Solution
|
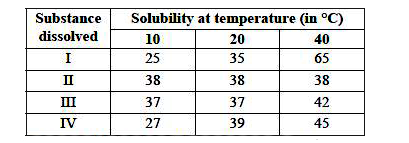
- Ankita tested the solubility of four different substances (I-IV) at di...
Text Solution
|
- Arrange the given substances in decreasing order of mass in grams. ...
Text Solution
|
- Refer to the given set of secondary sexual characteristics. I. Enlar...
Text Solution
|