The electronic structures of atoms W, X and Y are shown in the given figures.
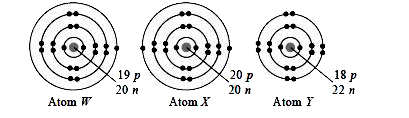
Study the structures carefully and select the correct statement(s) about these atoms.
I. Atoms X and Y will have same valency as their nucleon number is same.
II. Atoms Wand X are different isotopes of the same element as they have same number of neutrons.
III. Atoms W and Y will react to form `W_2Y`.
IV. Atoms X and Y are isobars as they have same nucleon number.