A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -NSO QUESTION PAPER 2018- 19 SET - A-ACHIEVERS SECTION
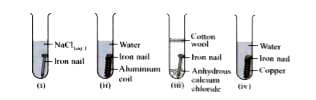
- Rishab has arranged the following set-ups to study the process of rust...
Text Solution
|
- Read the given passage and select the correct option for the following...
Text Solution
|
- Read the given passage and select the correct option for the following...
Text Solution
|
- Observe the given figure carefully. Select the correct statement...
Text Solution
|
- Refer to the given flow chart and select the correct option regarding ...
Text Solution
|
- The given graph shows the blood examination report of Mr X. What ...
Text Solution
|