A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Radioactivity)|78 VideosATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise Past Years Questions|67 VideosATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise Assertion and Reason|23 VideosALTERNATING CURRENT
ERRORLESS|Exercise Assertion & Reason|13 VideosCURRENT ELECTRICITY
ERRORLESS|Exercise ASSERTION AND REASON|26 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ATOMIC AND NUCLEAR PHYSICS-NCERT BASED QUESTIONS (Nucleus, Nuclear Reaction)
- In a nuclear reactor, moderators slow down the neutrons which come out...
Text Solution
|
- Energy generation in starts is mainly due to
Text Solution
|
- Which one of the following unclear reactions is a source of energy in ...
Text Solution
|
- Hydrogen bomb is based on which of the following phenomena ?
Text Solution
|
- If in a nuclear fusion process the masses of the fusing nuclei be m(1)...
Text Solution
|
- If 200 MeV energy is released in the fission of a single U^235 nucleus...
Text Solution
|
- Nuclear fission experiments show that the neutrons split the uranium n...
Text Solution
|
- Nuclear fusion is common to the pair
Text Solution
|
- The mass number of a nucleus is
Text Solution
|
- Heavy stable nuclei have more neutrons than protons. This is because o...
Text Solution
|
- Two nucleons are at a separation of 1 xx 10^-15 m. The net force betwe...
Text Solution
|
- Assertion: The whole mass of the atom is concentrated in the nucleus. ...
Text Solution
|
- O(2)molecules consists of two oxygen atoms. In the molecules , nuclear...
Text Solution
|
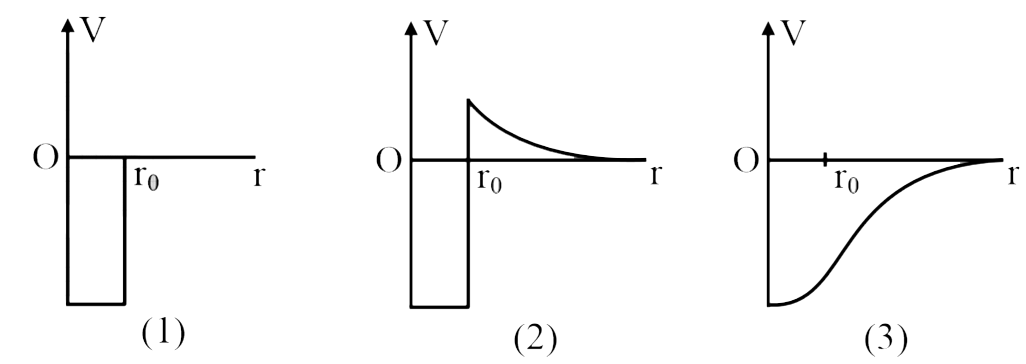
- Given below are three schematic graphs of potential energy V(r) versus...
Text Solution
|
- The mass number of He is 4 and that for suphur is 32. The radius of su...
Text Solution
|
- The radius of a nucleus of a mass number A is directly proportional to...
Text Solution
|
- If the nuclear radius of .^27 A1 is 3.6 Fermi, the approximate nuclear...
Text Solution
|
- The charge density in a nucleus varies with distance from the centre o...
Text Solution
|
- Order of magnitude of density of uranium nucleus is , [m = 1.67 xx 10^...
Text Solution
|
- A heavy nucleus at rest breaks into two fragments which fly off with v...
Text Solution
|