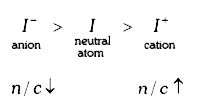A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Ionisation Energy)|54 VideosCLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Electron Affinity)|21 VideosCLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise Assertion & Reason |14 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -NCERT BASED QUESTIONS (Atomic and Ionic Radii)
- Ionic radii are
Text Solution
|
- Consider the isoelectronic species, Na^(+),Mg^(2+),F^(-) and O^(2-). T...
Text Solution
|
- The ionic radii (A^@)of C^(-4) and O^(-2) are 2.60 and 1.40 respective...
Text Solution
|
- The ionic conductance of following cation in a given concentration are...
Text Solution
|
- The correct order of increasing radii of the ions Br^(-), F^(-), O^(2-...
Text Solution
|
- Increasing order of ionic size : N^(3-),Na^(+),F^(-),O^(2-),Mg^(2+)
Text Solution
|
- Which of the following sets will have highest hydration energy and hig...
Text Solution
|
- Which of the following has the smallest size
Text Solution
|
- Which one is the correct order of the size of the iodine species ? A)...
Text Solution
|
- Which statement is correct
Text Solution
|
- Which one of the following should be most stable
Text Solution
|
- Smallest among these species is
Text Solution
|
- An atom of an element has electronic configuration 2,8,1. Which of the...
Text Solution
|
- The trivalent ion having size in lanthanide series is
Text Solution
|
- The atomic radii of the elements across the second period of the perio...
Text Solution
|
- Among the following, the most basic oxide is-
Text Solution
|
- Among the following, the set of isoelectronic ions is
Text Solution
|
- The isoelectronic pairs is :
Text Solution
|
- Mendeleev's periodic law states that the properties of element a...
Text Solution
|
- Among the elements Li, N ,C and Be one with the largest atomic radius ...
Text Solution
|