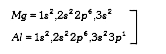A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Electron Affinity)|21 VideosCLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Electronegativity)|18 VideosCLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Atomic and Ionic Radii)|39 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -NCERT BASED QUESTIONS (Ionisation Energy)
- The successive ionzation enegry values for an element X are given belo...
Text Solution
|
- The set representing the correct order of the first ionisation potenti...
Text Solution
|
- The decreasing order of the ionization potential of the following elem...
Text Solution
|
- the highest ionisation energy stands for
Text Solution
|
- Which of the following electrons should generally have the highest val...
Text Solution
|
- Which of the following has lowest ionisation energy
Text Solution
|
- In view of their low ionisation energies, the alkali metals are
Text Solution
|
- The order of the magnitude of first ionisation potentials of Be,B,N an...
Text Solution
|
- Which has maximum first ionization potential?
Text Solution
|
- If the IP of Na is 5.48 eV, the ionsation potential of K will be
Text Solution
|
- Which of the following has the highest first ionisation energy?
Text Solution
|
- Which among the following elements have lowest value of IE1?
Text Solution
|
- In a given shell, the order of screeing effect is
Text Solution
|
- Among the following, the third ionisation energy is highest for
Text Solution
|
- Generally, the first ionisation energy increases along a period. But t...
Text Solution
|
- Which of the element is expected to have lowest first ionisation entha...
Text Solution
|
- The first ionisation potential of Na is 5.1eV. The value of electrons ...
Text Solution
|
- Amongest Be, B, Mg and Al, the second ionization potential is maximum ...
Text Solution
|
- In which of the following options the order of arrangement does ...
Text Solution
|
- One among the following is the incrorrect order of increasing ionisati...
Text Solution
|