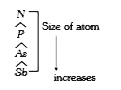A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise PAST YEARS QUESTIONS|33 VideosCLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise Assertion & Reason |14 VideosCLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Electronegativity)|18 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -NCERT BASED QUESTIONS (Valency and Oxidation State)
- Which of the following elements shows maximum number of different oxid...
Text Solution
|
- Which of the following element is found in its native state:-
Text Solution
|
- Which one of the elements with the following outer orbital configurati...
Text Solution
|
- Acidity of pentoxides in VA group
Text Solution
|
- Which of the following elements show maximum valency ?
Text Solution
|
- The correct order of increasing oxidising power
Text Solution
|
- In any period, the valency of an element with respect to oxygen
Text Solution
|
- which of the following gas does not have an octet or eight electrons i...
Text Solution
|
- the pentavalence in phosphorus is more stable as compared to that of n...
Text Solution
|
- Fluorine is the best oxidising agent because it has
Text Solution
|
- In the periodic table, the metallic character of elements
Text Solution
|
- In the periodic table, the basic character of oxides
Text Solution
|
- Elements A and B with their respective electronic configurations 3d^(...
Text Solution
|
- The stability of ions of Ge Sn and Pb will be in the order .
Text Solution
|
- Out of the following elements,which one is the most reactive chemicall...
Text Solution
|
- Thalium shows different oxidation states because
Text Solution
|
- The most stable oxidation state of thallium is +1
Text Solution
|
- An element X which occurs in the second short period has an outer elec...
Text Solution
|
- Which one of the following orders presents the correct sequence of the...
Text Solution
|
- Which of the following sequence correctly represents the decreasing ac...
Text Solution
|