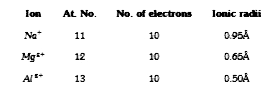A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -Assertion & Reason
- Assertion : Positive ions will be wider than parent atoms. Reason : ...
Text Solution
|
- Assertion : Dinegative anion of oxygen (O^(2-)) is quite common but di...
Text Solution
|
- Assertion: The atomic radii of calcium is smaller than sodium Reason...
Text Solution
|
- Assertion :First ionization energy is lower than oxygen. Reason :Ac...
Text Solution
|
- Assertion : Noble gases have maximum electron affinity. Reason : Hi...
Text Solution
|
- Assertion: E^(@) for Mn^(3+)//Mn^(2+) is more positive than Cr^(3+)//C...
Text Solution
|
- Assertion : Atomic number of the element ununbium is 112. Reason : ...
Text Solution
|
- Lanthanides and Actinides generally differ in
Text Solution
|
- Assertion: Shielding effect increases as we go down the group. Reaso...
Text Solution
|
- Assertion : More is the electron affinity greater is the reducing char...
Text Solution
|
- Assertion : I.E. of ""7N is more than that of ""8O as well as ""6C. ...
Text Solution
|
- Assertion : Electron affinity refers to an isolated atom’s attraction...
Text Solution
|
- Assertion: Mn atom loses ns electrons first during ionisation as compa...
Text Solution
|
- Assertion : In case of isoelectronic ions the ionic size increases wi...
Text Solution
|