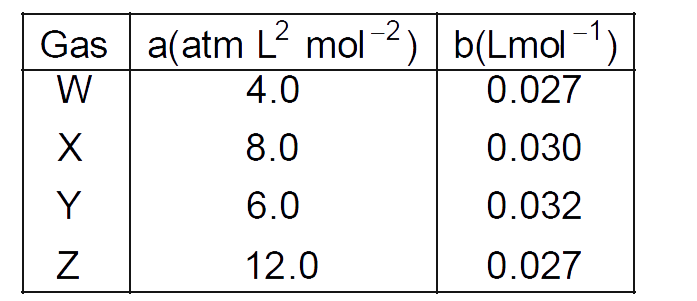A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
ERRORLESS|Exercise NCERT BASED QUESTIONS (VISCOSITY & SURFACE TENSION) |11 VideosSTATES OF MATTER
ERRORLESS|Exercise PAST YEAR QUESTIONS|34 VideosSTATES OF MATTER
ERRORLESS|Exercise NCERT BASED QUESTIONS (REAL GASES AND VAN DER WAALS EQUATION) |21 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|10 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-STATES OF MATTER -NCERT BASED QUESTIONS (CRITICAL STATE AND LIQUEFACTION OF GASES)
- A gas is liquefied
Text Solution
|
- 4.48 L of an ideal gas at STP requires 12 cal to raise its temperature...
Text Solution
|
- A gas can be liquefied at temperature T and pressure P provided "".
Text Solution
|
- Gases posses characteristic critical temperature which depends upon th...
Text Solution
|
- Adiabatic demagnetisation is atechnique used for
Text Solution
|
- The Van der Waal's parameters for gases W,X,Y and Z are- Which on...
Text Solution
|
- The critical temperatures of O(2),N(2),H(2) and CO(2) are 154.3K,126K,...
Text Solution
|
- The gas with the highest critical temperature is
Text Solution
|
- Which of the following is incorrect for critical temperature ?
Text Solution
|
- If the inversion temperature of a gas is -80^(@)C, then it will produc...
Text Solution
|
- The ratio gamma for inert gases is
Text Solution
|
- For the equation Cp - Cv = R, the significance of R is
Text Solution
|
- Consider the following statements (1) Joule-Thomson experiment is ...
Text Solution
|
- 2 mol He is mixed with 2 gm of H(2). The molar heat capacity at const...
Text Solution
|
- If helium is allowed to expand in vacuum, it liberates heat because
Text Solution
|
- Consider the following statements for diatomic gases, the ratio C(p) /...
Text Solution
|