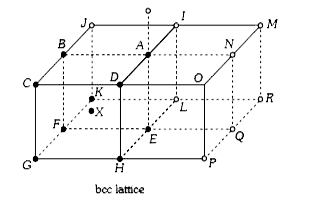
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
ERRORLESS|Exercise NCERT BASED QUESTIONS (CRYSTAL PACKING)|10 VideosSOLID STATE
ERRORLESS|Exercise NCERT BASED QUESTIONS (MATHEMATICAL ANALYSIS OF CUBIC SYSTEM AND BRAGG.S EQUATION) |13 VideosSOLID STATE
ERRORLESS|Exercise ASSERTION & REASON|11 VideosPOLYMERS
ERRORLESS|Exercise ASSERTION & REASON|4 VideosSOLUTIONS
ERRORLESS|Exercise ASSERTION AND REASON|16 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOLID STATE-NCERT BASED QUESTIONS (CRYSTALLOGRAPHY AND LATTICE)
- Which of the following is correct-
Text Solution
|
- How many space lattices are obtainable from the different crystal syst...
Text Solution
|
- Example of unit cell with crystallographic dimensions anebnec,alpha=ga...
Text Solution
|
- The number of close neighbours in a body-centred cubic unti cell of mo...
Text Solution
|
- Na and Mg crystallize in bcc- and fcc-type crystals, respectively, the...
Text Solution
|
- Potassium fluoride has NaCI type structure .What is the distance betwe...
Text Solution
|
- Which of the following hasno rotation of symmetry ?
Text Solution
|
- The lattice site in a pure crystal cannot be occupied by :
Text Solution
|
- The correct statement in the following is-
Text Solution
|
- The maximum ra dius of sphere that can be fitted in the octahedral hol...
Text Solution
|
- For tetrahedral coordination number, the radius ratio (r(c^(+)))/(r(a^...
Text Solution
|
- The number of next nearest neighbours around each particle in face-cen...
Text Solution
|
- If 'a' stands for the edge length of the cubic systems: simple cubic,b...
Text Solution
|
- An fcc unit cell of aluminium contains the equivalent of how many atom...
Text Solution
|
- Nickle crystallise in a fcc unit cell with a cell edge length of 0.352...
Text Solution
|
- Potassium crystallizes with a
Text Solution
|
- The molecule having three fold axis of symmetry is :
Text Solution
|
- The edge lengths of the unit cells in terms of the radius of spheres c...
Text Solution
|
- Space lattice of CaF(2) is
Text Solution
|