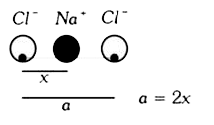A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
ERRORLESS|Exercise NCERT BASED QUESTIONS (DEFECTS IN CRYSTAL) |29 VideosSOLID STATE
ERRORLESS|Exercise PAST YEARS QUESTIONS|38 VideosSOLID STATE
ERRORLESS|Exercise NCERT BASED QUESTIONS (MATHEMATICAL ANALYSIS OF CUBIC SYSTEM AND BRAGG.S EQUATION) |13 VideosPOLYMERS
ERRORLESS|Exercise ASSERTION & REASON|4 VideosSOLUTIONS
ERRORLESS|Exercise ASSERTION AND REASON|16 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOLID STATE-NCERT BASED QUESTIONS (CRYSTAL STRUCTURE AND COORDINATION NUMBER)
- An example of fluorite structure is:
Text Solution
|
- The interionic distance for cesium chloride crystal will be
Text Solution
|
- In the distance between Na^(+) and CI^(-) ions in sodium chliride crys...
Text Solution
|
- A solid has a structure in which W atoms are located at the corners of...
Text Solution
|
- Body -centred cubic lattice has a corrdination number of
Text Solution
|
- A solid compound contains X,Y and Z atoms in a cubic lattice with X at...
Text Solution
|
- The unit cell of a binary compound of A and B metals has a ccp structu...
Text Solution
|
- Radius ratio of an ionic compound is 0.93. the structure of the above ...
Text Solution
|
- In the closest packed struture of a metallic lattice , the number of m...
Text Solution
|
- Structure of zinc blende|Structure of fluorite| Structure of anti fluo...
Text Solution
|
- The ionic radii of Rb^(+) and I^(-) are 1.46 and 2.16Å. The most proba...
Text Solution
|
- in which of the following structures coordination number for cati...
Text Solution
|
- What is the coordination number in a square close packed structures in...
Text Solution
|
- Which of the following statements is not true about NaCl structure?
Text Solution
|
- In CsCl structure, the coordination number of Cs^(+) is
Text Solution
|
- Point out the correct statement for the set of characteristics of ZnS ...
Text Solution
|
- The crystal structure of solid Mn(II) oxide is
Text Solution
|
- In Na2O, having antifluorite structure
Text Solution
|
- A unit cell of calcium fluoride has four calcium ions. The number of f...
Text Solution
|
- A mineral consists of a cubic close-packed structure formed by O^(2-) ...
Text Solution
|