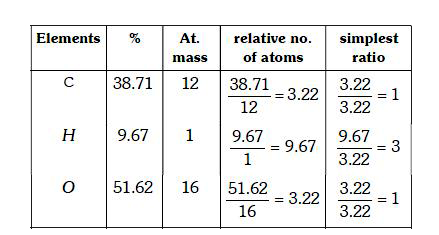
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|10 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS( CHEMICAL STOICHIOMETRY AND METHOD OF CONCENTRATION EXPRESSION)|33 VideosREDOX REACTIONS
ERRORLESS|Exercise ASSERTION & REASON|6 VideosSTATES OF MATTER
ERRORLESS|Exercise ASSERTION AND REASON|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOME BASIC CONCEPTS OF CHEMISTRY-PAST YEARS QUESTIONS
- A compound possesses 8% sulphur by mass. The least molecular mass is
Text Solution
|
- What is the mass of the precipitate formed when 50 mL of 16.9% solutio...
Text Solution
|
- The equivalent weight of phosphoric acid (H(3)PO(4)) in the reaction N...
Text Solution
|
- How many molecules are present in 1 kg of hydrogen ?
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- Which has the maximum number of molecules among the following ?
Text Solution
|
- If 10^(21) molecules are removed from 200 mg of CO(2), the number of m...
Text Solution
|
- what volume of hydrogen gas , at 273 K and 1 atm pressure will be con...
Text Solution
|
- The maximum amount of BaSO(4) precipitated on mixing BaCl(2) (0.5 M) w...
Text Solution
|
- Suppose the elements X and Y combine to form two compounds of XY(2) an...
Text Solution
|
- In which case is the number of molecules of water maximum?
Text Solution
|
- A compound contains atoms A, B and C. the oxidation number of A is +2,...
Text Solution
|
- 20.0 g of magnesium carbonate sample decomposes on heating to give car...
Text Solution
|
- An organic compound contains carbon, hydrogen and oxygen. Its elementa...
Text Solution
|
- During electrolysis of water, the volume of oxygen liberate is 2.24 dm...
Text Solution
|
- Under similar conditions of pressure and temperature, 40 ml of slightl...
Text Solution
|
- 1.0 g of magnesium is burnt with 0.56 g O(2) in a closed vessel. Which...
Text Solution
|
- If 30 ml of H2 and 20 ml of O2 react to form water, what is left at th...
Text Solution
|
- A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with c...
Text Solution
|
- Which one of the following has maximum number of atoms?
Text Solution
|