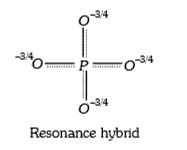
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Molecular Orbital Theory)|43 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Hydrogen Bonding)|22 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Polarisation and Fajan.s Rule)|14 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (Resonance)
- Which one in the following is not the resonance structure of CO(2)
Text Solution
|
- The bond order in CO3^(2-) ion between C - O is
Text Solution
|
- Which does not show resonance
Text Solution
|
- Write the resonance structure of CO(3)^(2-) and HCO(3)^(ө).
Text Solution
|
- Select the incorrect statement about the following :
Text Solution
|
- Resonance occurs due to the :
Text Solution
|
- In PO(4)^(3-) ion, the effective charge on each oxygen atom and P-O bo...
Text Solution
|
- Point out incorrect statement about resonance
Text Solution
|
- Cl-O bond order in perchlorate ion is
Text Solution
|
- In PO(4)^(3-) ion, the formal charge on the oxygen atom of P-O bond...
Text Solution
|