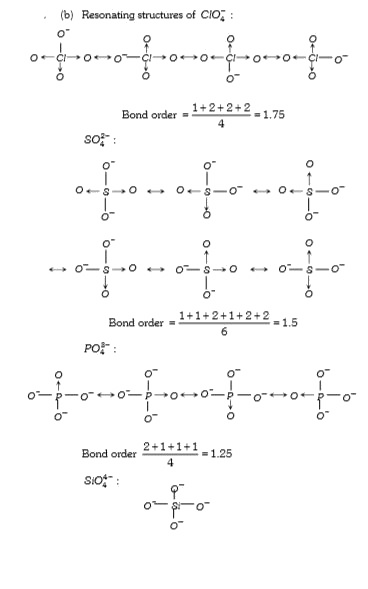
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Hydrogen Bonding)|22 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Types of Bonding and Forces in Solid)|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Resonance)|10 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (Molecular Orbital Theory)
- According to molecular orbital theory, the paramagnetism of O(2) molec...
Text Solution
|
- Which of the following is paramagnetic
Text Solution
|
- Which of the following molecules/ins does not contain unpaired electro...
Text Solution
|
- Using MOT, compare O2^(+) and O2^(-) species and choose the incorrect ...
Text Solution
|
- Which one of the following oxides is expected to exhibit paramagnetic ...
Text Solution
|
- N2 and O2 are converted into monoanions N2^- and O2^- respectively. Wh...
Text Solution
|
- Which one does not exhibit paramagnetism
Text Solution
|
- Substance which is weakly repelled by a magnetic field is
Text Solution
|
- Bond energies in NO,NO^(+) and NO^(-) are such as
Text Solution
|
- Which of the following order of energies of moleuclar orbitals of N...
Text Solution
|
- Which of the following statement is not correct from the view point of...
Text Solution
|
- Arrange the following ions in the order of decreasing X-O bond length ...
Text Solution
|
- The correct order of O-O bond length in O2,H2 O and O3.
Text Solution
|
- The correct order of increasing C - O bond length CO, CO3^(2-) , CO2 i...
Text Solution
|
- The bond order of super oxide ion O2^(2-) is
Text Solution
|
- Among the following, the species with the highest bond order is-
Text Solution
|
- The correct order of increasing C-O bond lengths in CO, CO3^(2-) and ...
Text Solution
|
- The diamagnetic species is
Text Solution
|
- In which of the following processes, the bond order has increased and ...
Text Solution
|
- During the change of O(2) to O(2)^(-), the incoming electron goes to t...
Text Solution
|