A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (METHODS OF EXPRESSING CONCENTRATION OF SOLUTION)|77 VideosSOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (COLLIGATIVE PROPERTIES)|8 VideosSOLID STATE
ERRORLESS|Exercise ASSERTION & REASON|11 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOLUTIONS -ASSERTION AND REASON
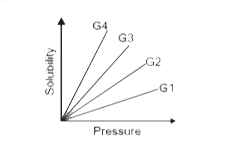
- The variation of solubility of four different gases (G1, G2, etc.) in ...
Text Solution
|
- Assertion :- If a liquid solute more volatile than the solvent is adde...
Text Solution
|
- Assertion : Azeotropic mixtures are formed only by non - ideal solutio...
Text Solution
|
- Assertion :- Molecular mass of polymers cannot be calculated using boi...
Text Solution
|
- Each question contains STATEMENT-I(Assertion) and STATEMENT-2(Reason)....
Text Solution
|
- Assertion (A): Camphor is used as a solvent in the determination of th...
Text Solution
|
- Assertion : If red blood cells were removed from the body and placed i...
Text Solution
|
- Assertion :- On adding NACl to water its vapour pressure increases. ...
Text Solution
|
- Assertion :Molar heat of vaporisation of water is greater than benzene...
Text Solution
|
- Assertion :- Ice melts faster at higher ltitude. Reason :- At high a...
Text Solution
|
- Assertion : Molecular mass of benzoic acid when determined by colligat...
Text Solution
|
- Assertion :- One molal aqueous solution of glucose contains 180g of gl...
Text Solution
|
- Assertion: C Cl(4)and H(2)Oare immiscible . Reason : C Cl(4) is a po...
Text Solution
|
- Assertion: Isotonic solutions do not show phenomenon of osmosis. Rea...
Text Solution
|
- Assertion (A): The increasing pressure on water decreases its freezing...
Text Solution
|
- Statement - The water pouch of instant cold pack for treating athlet...
Text Solution
|
- Assertion : The angle of contact of a lilquid with a solid decreases w...
Text Solution
|