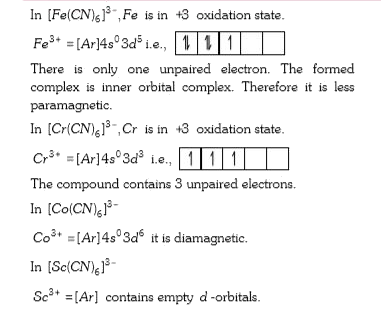
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (CRYSTAL FIELD THEORY)|17 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (COMPLEX STABILITY, SPECTROCHEMICAL SERIES AND EAN)|22 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (WERNER.S COORDINATION THEORY)|16 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-NCERT BASED QUESTION (VALENCE BOND THEORY AND GEOMETRY AND MAGNETIC NATURE OF COORDINATION COMPOUNDS)
- Which of the following is diamagnetic in nature ?
Text Solution
|
- The complesx [CoF(6)]^(4-) is
Text Solution
|
- Maximum value of paramagnetism is shown by
Text Solution
|
- A magnetic moment of 1.73 B.M. will be shown by one among the followin...
Text Solution
|
- The correct statement about the magnetic properties of [Fe(CN)(6)]^(3-...
Text Solution
|
- The spin only magnetic moment value of Cr(CO)(6) is
Text Solution
|
- The pair in which both species have same magnetic moment (spin only va...
Text Solution
|
- Wht will be the theoretical value of magnetic moment (mu) when CN^(-) ...
Text Solution
|
- Which of the following species will be diamagnetic
Text Solution
|
- The reaction [Fe(CNS)6]^(3-)rarr[FeF6]^(3-) takes place with:
Text Solution
|
- The complex showing a spin -magnetic momnet of 2.82 BM is .
Text Solution
|
- Which of the following complex ion is not expected to absorb visible l...
Text Solution
|
- Amongst [NiCl(4)]^(2-), [Ni(H(2)O)(6)]^(2+), [Ni(PPh(3))(2)Cl(2)], [Ni...
Text Solution
|
- What is the shape of Fe(CO)5
Text Solution
|
- What type of hybridization is involved in [Fe(CN)6]^(3-)
Text Solution
|
- Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati...
Text Solution
|
- Which of the following shell form an outer octahedral complex
Text Solution
|
- Which has square planar geometry
Text Solution
|
- Hybridization shape and magnetic moment of K(3)[Co(CO)(6)] is
Text Solution
|
- The correct statement with respect to the complexes Ni(CO)4 and [Ni(CN...
Text Solution
|