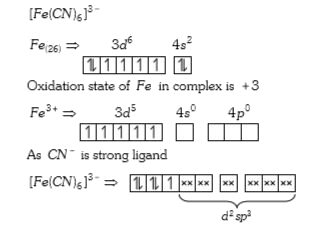
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (CRYSTAL FIELD THEORY)|17 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (COMPLEX STABILITY, SPECTROCHEMICAL SERIES AND EAN)|22 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (WERNER.S COORDINATION THEORY)|16 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-NCERT BASED QUESTION (VALENCE BOND THEORY AND GEOMETRY AND MAGNETIC NATURE OF COORDINATION COMPOUNDS)
- Amongst [NiCl(4)]^(2-), [Ni(H(2)O)(6)]^(2+), [Ni(PPh(3))(2)Cl(2)], [Ni...
Text Solution
|
- What is the shape of Fe(CO)5
Text Solution
|
- What type of hybridization is involved in [Fe(CN)6]^(3-)
Text Solution
|
- Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati...
Text Solution
|
- Which of the following shell form an outer octahedral complex
Text Solution
|
- Which has square planar geometry
Text Solution
|
- Hybridization shape and magnetic moment of K(3)[Co(CO)(6)] is
Text Solution
|
- The correct statement with respect to the complexes Ni(CO)4 and [Ni(CN...
Text Solution
|
- Which of the following molecules is not tetrahedral ?
Text Solution
|
- Geometrical shapes of the complex formed by the reaction of Ni^(2+) wi...
Text Solution
|
- In Fe(CO)(5), the Fe larr CO sigma bond results by the overlap between...
Text Solution
|
- The number of unpaired electrons in Ni (CO)(4) is
Text Solution
|
- Which complex compound obeys 18-electron rule
Text Solution
|
- Both [Ni(CO)4] and [Ni (CN)4]^(2-) are diamagnetic The hybridisation...
Text Solution
|
- Which of the following complex is an outer orbital complex?
Text Solution
|
- Which one of the following is an outer orbital complex and exhibits pa...
Text Solution
|
- The diamagnetic species is
Text Solution
|
- The bonds in K4[Fe(CN)6] are
Text Solution
|
- Amongst Ni(CO)(4),[Ni(CN)(4)]^(2-) and NiCl(4)^(2-):
Text Solution
|
- The geometry of Ni(CO)4 and Ni(PPH3)2Cl2 are
Text Solution
|