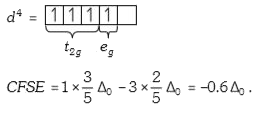A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (COMPLEX STABILITY, SPECTROCHEMICAL SERIES AND EAN)|22 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (PREPARATION AND APPLICATION OF COORDINATION COMPOUNDS)|11 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (VALENCE BOND THEORY AND GEOMETRY AND MAGNETIC NATURE OF COORDINATION COMPOUNDS)|48 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-NCERT BASED QUESTION (CRYSTAL FIELD THEORY)
- Which is high spin complex
Text Solution
|
- Which complex of Co^(2+) will have the weakest crystal field splittin...
Text Solution
|
- Which of the following configuration of ions has zero CFSE in both str...
Text Solution
|
- The complex ion having minimum magnitude of Delta0 (CFSE) is
Text Solution
|
- Which of the following complex ions is expected to absorb visible ligh...
Text Solution
|
- Among the following , the coloured compound is
Text Solution
|
- [Co(CN)(6)]^(3-), a complex ion of cobalt (III), absorbe radiations in...
Text Solution
|
- The crystal field stabilization energy (CFSE) is the highest for
Text Solution
|
- In which of the following coordination entities the magnitude of Delta...
Text Solution
|
- The colour of the coordination compounds depends on the crystal field ...
Text Solution
|
- The CFSE for octahedral [CoCl(6)]^(4-) is 18,000 cm^(-1). The CFSE for...
Text Solution
|
- The magnitude of crystal field stabilisation energy (CFSE of Delta(1))...
Text Solution
|
- Crystal field stabilization energy for high spin d^4 octahedral comple...
Text Solution
|
- Low spin complex of d^6-cation in an octahedral field will have the fo...
Text Solution
|
- Among the following complexes, the one which shows zero crystal field ...
Text Solution
|
- Which of the following configuration can undergo distortion
Text Solution
|
- The INCORRECT statement is :
Text Solution
|