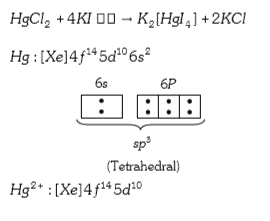A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (ORGANOMETALLIC COMPOUNDS)|13 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise PAST YEARS QUESTIONS|60 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (COMPLEX STABILITY, SPECTROCHEMICAL SERIES AND EAN)|22 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-NCERT BASED QUESTION (PREPARATION AND APPLICATION OF COORDINATION COMPOUNDS)
- Chlorophyll is a coordination compound having central atom of:
Text Solution
|
- Complex salt can be made by the combination of [Co^(III)(NH3)5Cl]^(x) ...
Text Solution
|
- AgCl dissolves in ammonia solution giving
Text Solution
|
- Which one of the following forms with an excess of CN^(-) (Cyanide) a ...
Text Solution
|
- In any ferric salt on adding potassinum ferrocyanide, a prussian blue ...
Text Solution
|
- Ferric ion forms a Prussian blue coloured precipitate due to fromation...
Text Solution
|
- Cold ferrous sulphate solution on absorption of NO develops brown colo...
Text Solution
|
- In the brown ring complex [Fe(H(2)O)(3)NO]SO(4) nitric oxide behaves ...
Text Solution
|
- Addition of excess potassium iodide solution to a solution of mercuri...
Text Solution
|
- In basic medium the amount of Ni^(2+) in a solution can be estimated w...
Text Solution
|
- The compound that inhibits the growth of tumors is:
Text Solution
|