A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-ALCOHOLS, PHENOLS AND ETHERS-Assertion & Reason
- Statement I: A triester of glycerol with stearic acid on boiling with ...
Text Solution
|
- Assertion : Phenol is a weak acid than ethanol. Reason : Groups wit...
Text Solution
|
- Assertion : The resonance structure of H-underset(H)underset(|)ove...
Text Solution
|
- Assertion : Phenol undergoes Kolbe's reaction whereas ethanol does ...
Text Solution
|
- Asserion: Lucas reagent is a micture of anhydrous ZnCI(2) and concentr...
Text Solution
|
- Assertion: Resorchiral turns FeCI(3) solution purple. Reason: Reasor...
Text Solution
|
- Assertion: Alcohol and ohenol can be distinguished by sodium hydroxide...
Text Solution
|
- Assertion : The major product formed by heating C(6)H(5)CH(2)OCH(3) wi...
Text Solution
|
- Assertion: The pK(a) of acetic acid is lower than that of phenol. R...
Text Solution
|
- Assertion : Alcoholic fermentation involves conversion of sugar into...
Text Solution
|
- Assertion : The water solubility of the alcohols follow the order t-...
Text Solution
|
- Acid catalysed dehydration of t-butanol is faster than that of n-butan...
Text Solution
|
- Assertion : Phenols cannot be converted into esters by direct reacti...
Text Solution
|
- Assertion: Alcohols are easily protonated thena phenols. Reason: Alc...
Text Solution
|
- Assertion : Phenol is a weak acid than ethanol. Reason : Groups wit...
Text Solution
|
- Assertion : (CH(3))(3)C-Br and CH(3)CH(2)O"N"a react to form (CH(3))(3...
Text Solution
|
- Assertion : Rate of hydrolysis of methyl chloride to methanol is highe...
Text Solution
|
- Assertion. t-Butyl Methyl ether is not prepared by the reaction of t-b...
Text Solution
|
- Assertion: The ease of dehydration of the following alcohols is R...
Text Solution
|
- Assertion: Alcohols have higher boiling points than ethers of comparab...
Text Solution
|
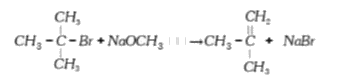 It is because alkoxides are not only nucleophiles, but also strong bases as well. They react with alkyl halides leading to elimination reaction.
It is because alkoxides are not only nucleophiles, but also strong bases as well. They react with alkyl halides leading to elimination reaction.