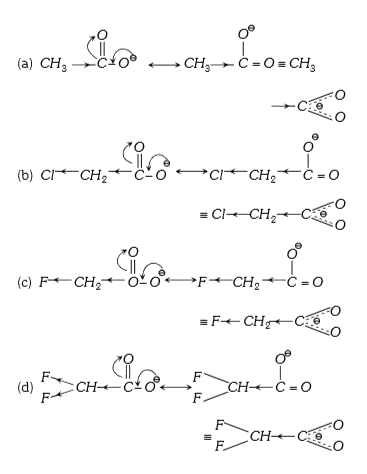
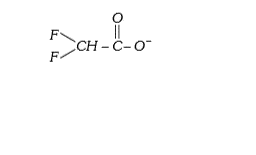A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Organic Reactions and their Mechanism )|46 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Structural and Stereo Isomerism )|99 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 VideosHYDROCARBONS
ERRORLESS|Exercise ASSERTION & REASON|18 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-GENERAL ORGANIC CHEMISTRY -NCERT BASED QUESTIONS (Dipole Moment, Resonance and Reaction Intermediates )
- Amongst the given structures , which are permissible resonance forms ?
Text Solution
|
- In which of the following compounds the carbon marked with asterisk is...
Text Solution
|
- Ionic species are stabilised by the dispersal of charge. Which of the ...
Text Solution
|
- The strongest acid is :
Text Solution
|
- Aromatic properties of benzene are proved by
Text Solution
|
- Carboxylic acids are easily ionised. The main reason of this statement
Text Solution
|
- Which among the following statements are true with respect to electron...
Text Solution
|
- Which of the following is observed in ethylene molecule
Text Solution
|
- Which of the following is the most stable compound?
Text Solution
|
- The compound which gives the most stable carbonium ion on dehydration ...
Text Solution
|
- The stability of Me(2)C=CH(2) is more than that of MeCH(2)CH=CH(2) due...
Text Solution
|
- Which species are more resonance stabilized in the following pairs: ...
Text Solution
|
- C-Cl bond in CH(2) = CH - Cl is difficult to cleave due to
Text Solution
|
- the ascending order of stability of the carbanion CH(3)(P),C(6)H(5)ove...
Text Solution
|
- For the given four structures , I to IV, which of the following is cor...
Text Solution
|
- Arrange the following free radiacals in order of decreasing stability....
Text Solution
|
- Stability of iso-butylene can be best explained by
Text Solution
|
- Compound which shows positive mesomeric effect
Text Solution
|
- Among the following, which is least acidic ?
Text Solution
|
- Given The decreasing order of the acidic character is
Text Solution
|