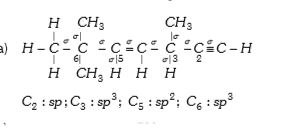A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Structural and Stereo Isomerism )|99 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 VideosHYDROCARBONS
ERRORLESS|Exercise ASSERTION & REASON|18 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-GENERAL ORGANIC CHEMISTRY -PAST YEARS QUESTIONS
- The state of hybridization of C(2),C(3),C(5) and C(6) of the hydrocarb...
Text Solution
|
- 1,2-butadiene has
Text Solution
|
- What is the hybridisation state of benzyl carbonium ion
Text Solution
|
- The total number of pi - bond electrons in the following structure is
Text Solution
|
- C-C bond length in benzene is
Text Solution
|
- The correct order of increasing bond length of C - H ,C - O, C - C and...
Text Solution
|
- Examine the following common chemical structures to which simple funct...
Text Solution
|
- Considering the state of hybridization of carbon atoms, find out the m...
Text Solution
|
- Which of the following are correct chain isomers of butane ? (i) <im...
Text Solution
|
- The pair of electron in the given carbanion, CH(3)C -= C^(Θ), is prese...
Text Solution
|
- Which of the following C-H bond has the lowest bond dissociation energ...
Text Solution
|
- The number of sigma (sigma) and pi(pi) bonds in pent-2-en-4-yne is:
Text Solution
|
- The dipole moment is the highest for
Text Solution
|
- Base strength of (1) H(3)Coverset(Theta)(C)H(2), (2) H(2)C=overset(...
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- Which one of the following orders is correct regarding the inductive e...
Text Solution
|
- Which one of the following compounds will be most readily dehydrated?
Text Solution
|
- Choose the correct order of stability of carbocation using concept of ...
Text Solution
|
- Homolytic fission of the following alkanes forms free radicals CH(3) -...
Text Solution
|
- Consider the following compound. Hyperconjugation occurs in
Text Solution
|