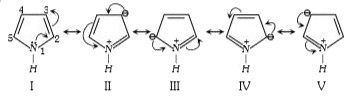
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Structural and Stereo Isomerism )|99 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 VideosHYDROCARBONS
ERRORLESS|Exercise ASSERTION & REASON|18 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-GENERAL ORGANIC CHEMISTRY -PAST YEARS QUESTIONS
- Which one of the following compounds is most acidic
Text Solution
|
- Among the following the dissociation constant is highest
Text Solution
|
- How many lines of symmetry does the above figure have ? <img src="ht...
Text Solution
|
- Which of the following statements is not correct for a nucleophile ?
Text Solution
|
- For the following (i) I^(-) (ii) Cl^(-) (iii) Br^(-) the increasin...
Text Solution
|
- The order of decreasing reactivity towards an electrophilic reagent, f...
Text Solution
|
- Which one of the following is most reactive towards electrophilic reag...
Text Solution
|
- Which of the following species is not electrophilic in nature
Text Solution
|
- Among the following compounds the one that is most reactive towards el...
Text Solution
|
- In which of the following compounds, the C-Cl bond ionisation shall gi...
Text Solution
|
- Which of the following are correct chain isomers of butane ? (i) <im...
Text Solution
|
- In a reaction of C(6)H(5)Y the major product (gt60%) is m-isomer, so t...
Text Solution
|
- Which of the following is not an electrophile
Text Solution
|
- How many lines of symmetry does the above figure have ? <img src="ht...
Text Solution
|
- The correct statement regarding electrophile is
Text Solution
|
- Which of the following is correct with respect to -I effect of the sub...
Text Solution
|
- Which of the following carbocations is expected to be most stable ?
Text Solution
|
- The compound that is most difficult to protonate is
Text Solution
|
- In the chemical reactions, the compounds 'A' and 'B' respectively...
Text Solution
|
- Nucleophilic addition reaction will be most favoured in
Text Solution
|