Topper's Solved these Questions
JEE MAINS 2022
JEE MAINS PREVIOUS YEAR|Exercise PHYSICS (SECTION B)|1 VideosJEE MAINS 2022
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY (SECTION A)|19 VideosJEE MAINS 2022
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY (SECTION-B)|10 VideosJEE MAINS 2021
JEE MAINS PREVIOUS YEAR|Exercise Chemistry (Section B )|10 VideosJEE MAINS 2023 JAN ACTUAL PAPER
JEE MAINS PREVIOUS YEAR|Exercise Question|360 Videos
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2022-Chemistry-Section B
- The osmotic pressure of blood is 7.47 bar at 300 K. To inject glucose ...
Text Solution
|
- The cell potential for the following cell Pt |H2(g)|H+ (aq)|| Cu^(2+) ...
Text Solution
|
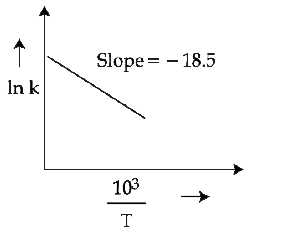
- The rate constants for decomposition of acetaldehyde have been measure...
Text Solution
|
- The difference in oxidation state of chromium in cliromate and dichrom...
Text Solution
|
- In the cobalt-carbonyl complex : [Co2(CO)g] , number of Co-Co bonds is...
Text Solution
|
- A 0.166 g sample of an organic compound was digested with conc. H2SO4 ...
Text Solution
|
- Number of electrophillic centres in the given compound is
Text Solution
|
- The major product'A' of the following given reaction has sp^2 hybridi...
Text Solution
|
- For complete combusion of methanol CH(3)OH(I) + (3)/(2) O(2)to CO(2)...
Text Solution
|
- A 0.5 percent solution of potassium chloride was found to freeze at ...
Text Solution
|
- 50 ML of 0.1 M of CH(3)COOH is being titrated against 0.1 M NaOH. When...
Text Solution
|
- A flask is filled with equal moles of A and B . The half lives of A a...
Text Solution
|
- 2.0 g of H(2) gas is adsrobed on 2.5 g of platinum powder at 300 K and...
Text Solution
|
- The spin - only magnetic moment value of the most basic oxide of vanad...
Text Solution
|
- The spin-only magnetic moment value of an octahedral complex among CoC...
Text Solution
|
- On complete combustion 0.30 g of an organic compound gave 0.20 g of ca...
Text Solution
|
- Compound 'P' on nitration with dil. HNO(3) yields to isomers (A) and (...
Text Solution
|
- The number of oxygen present in a nucleotide formed from a base, that ...
Text Solution
|
- Metal deficiency defect is shown by Fe(0.93)O . In the crystal, some F...
Text Solution
|
- If the uncertainty in velocity and position of a minute particle in sp...
Text Solution
|