Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-SAMPLE PAPER 2023 TERM I-SECTION - E
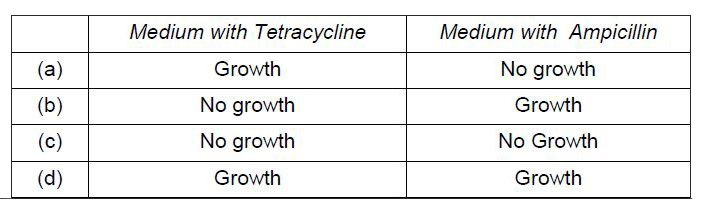
- The figure below shows the structure of a plasmid. A foreign DNA wa...
Text Solution
|
- Trace the events from copulation to zygote formation in a human female...
Text Solution
|
- Trace the development of a megaspore mother cell of a flower into a ma...
Text Solution
|
- Observe the segment of mRNA given below. Explain and illustrate ...
Text Solution
|
- Observe the segment of mRNA given below. Gene encoding RNA Polym...
Text Solution
|
- Study the schematic representation of the genes involved in the lac op...
Text Solution
|
- Study the schematic representation of the genes involved in the lac op...
Text Solution
|
- Study the schematic representation of the genes involved in the lac op...
Text Solution
|
- Oil spill is a major environmental issue. It has been found that diffe...
Text Solution
|
- Oil spill is a major environmental issue. It has been found that diffe...
Text Solution
|
- Oil spill is a major environmental issue. It has been found that diffe...
Text Solution
|
- Insects in the Lepidopteran group lay eggs on maize crops. The larvae ...
Text Solution
|
- Insects in the Lepidopteran group lay eggs on maize crops. The larvae ...
Text Solution
|
- Insects in the Lepidopteran group lay eggs on maize crops. The larvae ...
Text Solution
|
 A foreign DNA was ligated at BamH1. The transformants were then grown in a medium containing antibiotics tetracycline and ampicillin. Choose the correct observation for the growth of bacterial colonies from the given table
A foreign DNA was ligated at BamH1. The transformants were then grown in a medium containing antibiotics tetracycline and ampicillin. Choose the correct observation for the growth of bacterial colonies from the given table