Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2023 JAN ACTUAL PAPER-Question
- Statement I: For colloidal particles, the values of colligative proper...
Text Solution
|
- A' and 'B' formed in the following set of reactions are:
Text Solution
|
- Uracil is a base present in RNA with the following structure. % of N i...
Text Solution
|
- If wavelength of the first line of the Paschen series of hydrogen atom...
Text Solution
|
- At 298 K, a 1 litre solution containing 10 mmol of Cr2O7^(2–) and 100 ...
Text Solution
|
- The dissociation constant of acetic acid is x ×10^(–5). When 25 mL of ...
Text Solution
|
- For independent processes at 300 K The number of non spontaneous ...
Text Solution
|
- Number of moles of AgCl formed in the following reaction is
Text Solution
|
- The d-electronic configuration of [CoCl4]^(2–) in tetrahedral crystal ...
Text Solution
|
- 5 g of NaOH was dissolved in deionized water to prepare a 450 mL stock...
Text Solution
|
- The number of correct statement/s from the following is A. Larger th...
Text Solution
|
- When Fe(0.93)O is heated in presence of oxygen, it converts to Fe2O3. ...
Text Solution
|
- What is the electron gain enthalpy for noble gases?
Text Solution
|
- The radius of the 2^(nd) orbit of Li^(2+) is x. The expected radius of...
Text Solution
|
- The compound which will have the lowest rate towards nucleophilc aroma...
Text Solution
|
- Reaction of thionyl chloride with white phosphorus forms a compound [A...
Text Solution
|
- The correct sequence of reagents for the preparation of Q and R is:
Text Solution
|
- Match the List-I with List-II,
Text Solution
|
- Compound A reacts with NH4Cl and forms a compound B. Compound B reacts...
Text Solution
|
- The correct order in aqueous medium of basic strength in case of methy...
Text Solution
|
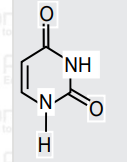 Given:Molar mass `N = 14g mol^(–1),O = 16 g mol^(–1),C = 12 g mol^(–1),H = 1 g mol^(–1)`
Given:Molar mass `N = 14g mol^(–1),O = 16 g mol^(–1),C = 12 g mol^(–1),H = 1 g mol^(–1)`