Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2023 JAN ACTUAL PAPER-Question
- A cubic solid is made up of two elements X and Y. Atoms of X are prese...
Text Solution
|
- A litre of buffer solution contains 0.1 mole of each of NH3 and NH4Cl ...
Text Solution
|
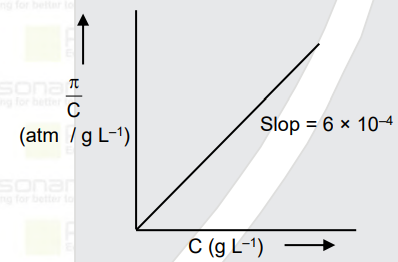
- The osmotic pressure of solutions of PVC in cyclohexanone at 300 K are...
Text Solution
|
- For the first order reaction A rarrB, the half life is 30 min. The tim...
Text Solution
|
- Consider the cell Pt(s) | H2(g) (1 atm) | H^+(aq, [H^+] = 1) || Fe^(3+...
Text Solution
|
- In sulphur estimation, 0.471 g of an organic compound gave 1.4439 g of...
Text Solution
|
- How many of the following metal ions have similar value of spin only m...
Text Solution
|
- The density of a monobasic strong acid (Molar mass 24.2 g/mol) is 1.21...
Text Solution
|
- The total number of lone pairs of electrons on oxygen atoms of ozone i...
Text Solution
|
- An athlete is given 100 g of glucose (C6H(12)O6) for energy. This is e...
Text Solution
|
- The number of paramagnetic species from the following is [Ni(CN)4]^(...
Text Solution
|
- Given below are two statements: Statement I: In froth floatation met...
Text Solution
|
- Match List I with List II Choose the correct answer from the opti...
Text Solution
|
- A.Ammonium salts produce haze in atmosphere. B.Ozone gets produced w...
Text Solution
|
- Match List I with List II
Text Solution
|
- Find out the major product from the following reaction
Text Solution
|
- Given below are two statements,one is labelled as Assertion A and othe...
Text Solution
|
- ‘A’ in the given reaction is
Text Solution
|
- Match List I with List II Choose the correct answer from the opti...
Text Solution
|
- Given below are two statements,one is labelled as Assertion A and othe...
Text Solution
|