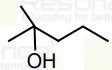
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2023 JAN ACTUAL PAPER-Question
- The correct order of pK(a) values for the following compounds is :
Text Solution
|
- Given below are two statements: One is labelled as Assertion A and the...
Text Solution
|
- Given below are two statements: One is labelled as Resertion A and the...
Text Solution
|
- Given below are two statements: Statement : During Electrolytic refini...
Text Solution
|
- The most stable carbocation for the following is:
Text Solution
|
- Decreasing order towards SN1 reaction for the following compounds is :
Text Solution
|
- Bond dissociation energy of E-H^(*) bond of the order. A. O B. S C. S...
Text Solution
|
- Maximum number of electrons that can be accommodated in shell with n =...
Text Solution
|
- In the above conversion of compound (X) to product (Y), the sequence o...
Text Solution
|
- Match List I with List Il:
Text Solution
|
- Match List I with List Il:
Text Solution
|
- Formulae for Nessler’s reagent is :
Text Solution
|
- 1L,0.02M solution of [Co(NH(3))(5)SO(4)] Br is mixed with 1L,0.02M sol...
Text Solution
|
- The Wave function (Psi) of 2s is given by: Psi(2s)=(1)/(2sqrt2pi)(1/...
Text Solution
|
- Which of the following reaction is correct?
Text Solution
|
- Chlorides of which metal are soluble in organic solvents:
Text Solution
|
- The strength of 50 volume solution of hydrogen peroxide is Given: Mola...
Text Solution
|
- Lead storage battery contains 38% by weight solution of H(2)SO(4).The ...
Text Solution
|
- The electrode potential of the following half cell at 298K X|X^(2+)(0...
Text Solution
|
- The graph of log((x)/(M)) vs log p for an adsorption process is a stra...
Text Solution
|
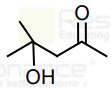 can be easily reduced using Zn-Hg/HCl to
can be easily reduced using Zn-Hg/HCl to