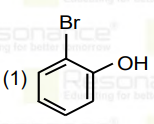
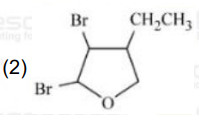
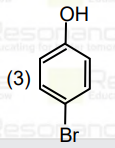
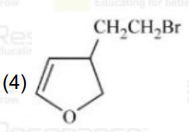
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2023 JAN ACTUAL PAPER-Question
- Number of compounds from the following which will not dissolve in cold...
Text Solution
|
- Iron (II) oxide has a cubic structure and each unit cell has side 5 Å...
Text Solution
|
- An organic compound 'A' with emperical formula C(6)H(6)O gives sooty f...
Text Solution
|
- What transition in the hydrogen spectrum would have the same wavelen...
Text Solution
|
- Choose the correct set of reagents for the following conversion. trans...
Text Solution
|
- Which one of the following statements is correct for electrolysis of b...
Text Solution
|
- Nd^(2+)=""
Text Solution
|
- When Cu^(2+) ion is treated with KI,a white precipitate, X appears in ...
Text Solution
|
- Identify X,Y and Z in the following reaction.(Equation not balanced) C...
Text Solution
|
- Consider the above reactions and identify the product B
Text Solution
|
- Consider the following reaction The correct statement for product B...
Text Solution
|
- H(2)O(2) acts as a reducing agent in
Text Solution
|
- Adding surfactants in non polar solvent,the micelles structure will lo...
Text Solution
|
- The methods NOT involved in concentration of ore are A.Liquid B.Leac...
Text Solution
|
- Which of the following artificial sweeteners has the highest sweetness...
Text Solution
|
- Match List I with List II Choose the correct answer from the options...
Text Solution
|
- A protein'X'with molecular weight of 710,000u,on hydrolysis gives amin...
Text Solution
|
- Match items of column I and II Match List I with List II
Text Solution
|
- Cobalt chloride when dissolved in water forms pink colored complex X w...
Text Solution
|
- The correct order of melting points of dichlorobenzene is
Text Solution
|