A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2023 JAN ACTUAL PAPER-Question
- Consider the following reaction The correct statement for product B...
Text Solution
|
- H(2)O(2) acts as a reducing agent in
Text Solution
|
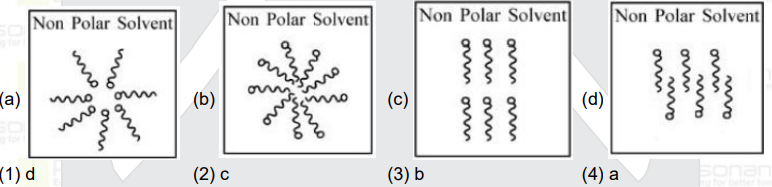
- Adding surfactants in non polar solvent,the micelles structure will lo...
Text Solution
|
- The methods NOT involved in concentration of ore are A.Liquid B.Leac...
Text Solution
|
- Which of the following artificial sweeteners has the highest sweetness...
Text Solution
|
- Match List I with List II Choose the correct answer from the options...
Text Solution
|
- A protein'X'with molecular weight of 710,000u,on hydrolysis gives amin...
Text Solution
|
- Match items of column I and II Match List I with List II
Text Solution
|
- Cobalt chloride when dissolved in water forms pink colored complex X w...
Text Solution
|
- The correct order of melting points of dichlorobenzene is
Text Solution
|
- The correct increasing order of the ionic radii is
Text Solution
|
- .The correct order of basicity of oxides of vanadium is
Text Solution
|
- .For reaction : SO(2)(g)+(1)/(2)O(2)(g) iff SO(3)(g) K(p)=2times10^(1...
Text Solution
|
- The rate constants of the above reaction at 200K and 300K are 0.03min^...
Text Solution
|
- The oxidation state of phosphorus in hypophosphoric acid is +
Text Solution
|
- The total pressure of a mixture of non-reacting X(0.6g) and Y(0.45g) i...
Text Solution
|
- At 27^(@)C,a solution containing 2.5g of solute in 250.0mL of solution...
Text Solution
|
- On complete combustion. 0.492g of an organic compound gave 0.792g of C...
Text Solution
|
- Zinc reacts with hydrochloric acid to give hydrogen and zinc chloride....
Text Solution
|
- The logarithm of equilibrium constant for the reaction Pd^(2+)+4Cl iff...
Text Solution
|
 (Surfactant structure) head tatal head tail
(Surfactant structure) head tatal head tail