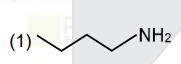
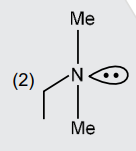
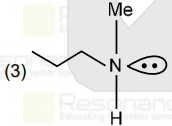
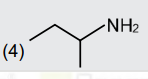
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2023 JAN ACTUAL PAPER-Question
- Evaluate the following statements for their correctness. A.The elevat...
Text Solution
|
- Given below are two statements : Statement I : Upon heating a borax b...
Text Solution
|
- An organic compound [A](C(4)H(11)N),shows optical activity and gives N...
Text Solution
|
- Compound A,C(3)H(10)O(5),given a tetraacetate with AC(2)O and oxidatio...
Text Solution
|
- Given below are two statements : Statement I : H(2)O(2) is used in th...
Text Solution
|
- A hydrocarbon 'X' with formula C(6)H(8) uses two moles of H(2) on cata...
Text Solution
|
- Which of the following elements have half-filled f-orbitals in their g...
Text Solution
|
- The Lewis acid character of boron tri halides follows the order :
Text Solution
|
- The normal rain water is slightly acidic and its pH value is 5.6 becau...
Text Solution
|
- Which one of the following statements is incorrect ?
Text Solution
|
- The element playing significant role in neuromuscular function and int...
Text Solution
|
- Enthalpies of formation of CCl(4)(g),H(2)O(g),CO(2)(g) and HCl are -10...
Text Solution
|
- If the CFSE of Ti(H(2)O(6))]^(3+) is -96.0 kJ/mol,this complex will ab...
Text Solution
|
- The number of alkali metal (s),from Li,K,Cs,Rb having ionization entha...
Text Solution
|
- At 298K,the solubility of silver chloride in water is 1.434 times10^(-...
Text Solution
|
- A sample of a metal oxide has formula M(0.83)O(1.00).The metal M can e...
Text Solution
|
- The number of molecules which gives haloform test among the following ...
Text Solution
|
- Amongst the following,the number of species having the linear shape is...
Text Solution
|
- The rate constant for a first order reaction is 20min^(-1).The time re...
Text Solution
|
- Assume carbon burns according to following equation : 2C(s)+O(2)(g) r...
Text Solution
|