A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2023 JAN ACTUAL PAPER-Question
- Decreasing order of dehydration of the following alcohols is
Text Solution
|
- The correct representation in six membered pyranose form for the follo...
Text Solution
|
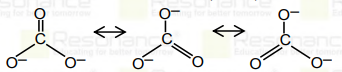
- Resonance in carbonate ion (CO(3)^(2-))is Which of the following is...
Text Solution
|
- Given below are two statements: one is labelled as Assertion A and the...
Text Solution
|
- Which of the following represents the lattice structure of A(0.95)O co...
Text Solution
|
- How can photochemical smog be controlled?
Text Solution
|
- Match List I with List II : Choose the correct answer from the option...
Text Solution
|
- Match List I with List II : Choose the correct answer from the option...
Text Solution
|
- Highest oxidation state of Mn is exhibited in Mn(2)O(7).The correct st...
Text Solution
|
- Given below are two statements: Statement I: Chlorine can easily combi...
Text Solution
|
- Given below are two statements: one is labelled as Assertion A and the...
Text Solution
|
- Given below are two statements: One is labelled as Assertion A and the...
Text Solution
|
- In the following reaction, ‘A’ is
Text Solution
|
- Choose the correct statement(s): A. Beryllium oxide is purely acidic ...
Text Solution
|
- But-2-yne is reacted separately with one mole of Hydrogen as shown bel...
Text Solution
|
- The density of 3M solution of NaCl is 1.0gmL^(-1).Molality of the solu...
Text Solution
|
- Sum of oxidation states of bromine in bromic acid and perbromic acid i...
Text Solution
|
- 25mL of an aqueous solution of KCl was found to require 20mL of 1M AgN...
Text Solution
|
- A and B are two substances undergoing radioactive decay in a container...
Text Solution
|
- (i) X(g) iff Y(g) + Z(g) , K(p1)=3 (ii) A(g) iff 2B(g), K(p2)=3 If the...
Text Solution
|