A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2023 JAN ACTUAL PAPER-Question
- The starting material for convenient preparation of deuterated hydroge...
Text Solution
|
- The effect of addition of helium gas to the following reaction in equi...
Text Solution
|
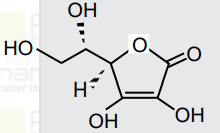
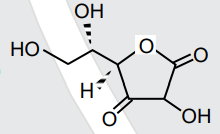
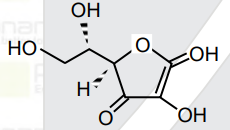
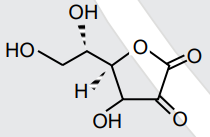
- All structures given below are of vitamin C. Most stable of them is :
Text Solution
|
- Which one of the following sets of ions represents a collection of iso...
Text Solution
|
- The correct order of bond enthalpy (kJ mol^(–1)) is :
Text Solution
|
- The complex cation which has two isomers is :
Text Solution
|
- The structures of major products A, B and C in the following reaction ...
Text Solution
|
- Which element is not present in Nessler's reagent ?
Text Solution
|
- The graph which represents the following reaction is :(C6H5)2C-Clover...
Text Solution
|
- Given below are two statements : one is labelled as Assertion (A) and ...
Text Solution
|
- Given below are two statements : one is labelled as Assertion (A) and ...
Text Solution
|
- For electron gain enthalpies of the elements denoted as Delta(eg)H, th...
Text Solution
|
- In a reaction reagents 'X' and 'Y' respectively are :
Text Solution
|
- Given below are two statements : one is labelled as Assertion (A) and ...
Text Solution
|
- Given below are two statements : Statement I : Sulphanilic acid gives...
Text Solution
|
- In figure, a straight line is given for Freundrich Adsorption (y = 3x ...
Text Solution
|
- O – O bond length in H2O2 is X than the O -O bond length in F2O2. The ...
Text Solution
|
- Among the following, the number of tranquilizer/s is/are .
Text Solution
|
- 20% of acetic acid is dissociated when its 5g is added to 500 mL of wa...
Text Solution
|
- The molality of a 10% (v/v) solution of di-bromine solution in "CC"l4 ...
Text Solution
|