XII BOARDS PREVIOUS YEAR-QUESTION PAPER 2023-Question
- (a)What type of deviation from Raoult's law is shown by a mixture of e...
Text Solution
|
- An alkyl halide (A) of molecular formula C6H(13)Cl on treatment with a...
Text Solution
|
- The rate constant for the first order deomposition of N2O5 is given by...
Text Solution
|
- Name the cell which: (a) was used in Apollo Space programme.. (b) is ...
Text Solution
|
- Write IUPAC names of the follwing coorrdination entites: (a) [Cr(NH3)3...
Text Solution
|
- Which of the following is an appropriate set of reactants for the prep...
Text Solution
|
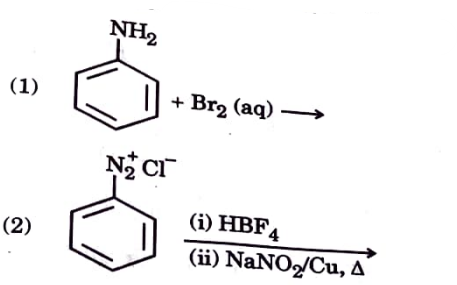
- (a) Write the prouducts of the following reaction (b) Do the follo...
Text Solution
|
- The following data were odtained during the frist order thermal decomp...
Text Solution
|
- If benzoic acid (M=122 g mol^-1) is associated into a dimer when disso...
Text Solution
|
- Answer any three of the following question : (a) Explain the type of ...
Text Solution
|
- Account for the following : (a) Benzyl chloride is highly reactive tow...
Text Solution
|
- (a) (i) Write hydroboration-oxidation reaction with an example. (ii)...
Text Solution
|
- (b) (i) What happens when phenol reacts with (1) Conc HNO3 and (2) C...
Text Solution
|
- Amines are usually formed from nitro componds, halides, amides, imides...
Text Solution
|
- Living system are made of various complex biomolecules like carbohydra...
Text Solution
|
- (a) Draw structure of the 2,4-dinitrophenylhydrazone of benzaldehyde ...
Text Solution
|
- Calculate the emf of the following cell at 298 K : Al(s) |Al^(3+)(0.00...
Text Solution
|
- (b) (i) The molar conductivities of NH4^+ and Cl^- ion are 73.8 S cm^2...
Text Solution
|
- (a) (i) Account for the following: (1) Zn^(2+) salts are colourless w...
Text Solution
|
- (b) (i) Name two oxometal anions of the 3d series of the transition me...
Text Solution
|