A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NEET
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise QUESTION|92 VideosNEET
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise MCQ|94 VideosNEET
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise MCQ|94 VideosMETALLURGICAL OPERATIONS
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise MCQ|21 VideosNEET 2018
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise QUESTION|45 Videos
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-NEET-CHEMISTRY
- With Which one of the following elements silicon should be doped so as...
Text Solution
|
- H(3)C-underset(CH(3))underset(|)(CH)-CH=CH(2)+HBr to A. A is predomi...
Text Solution
|
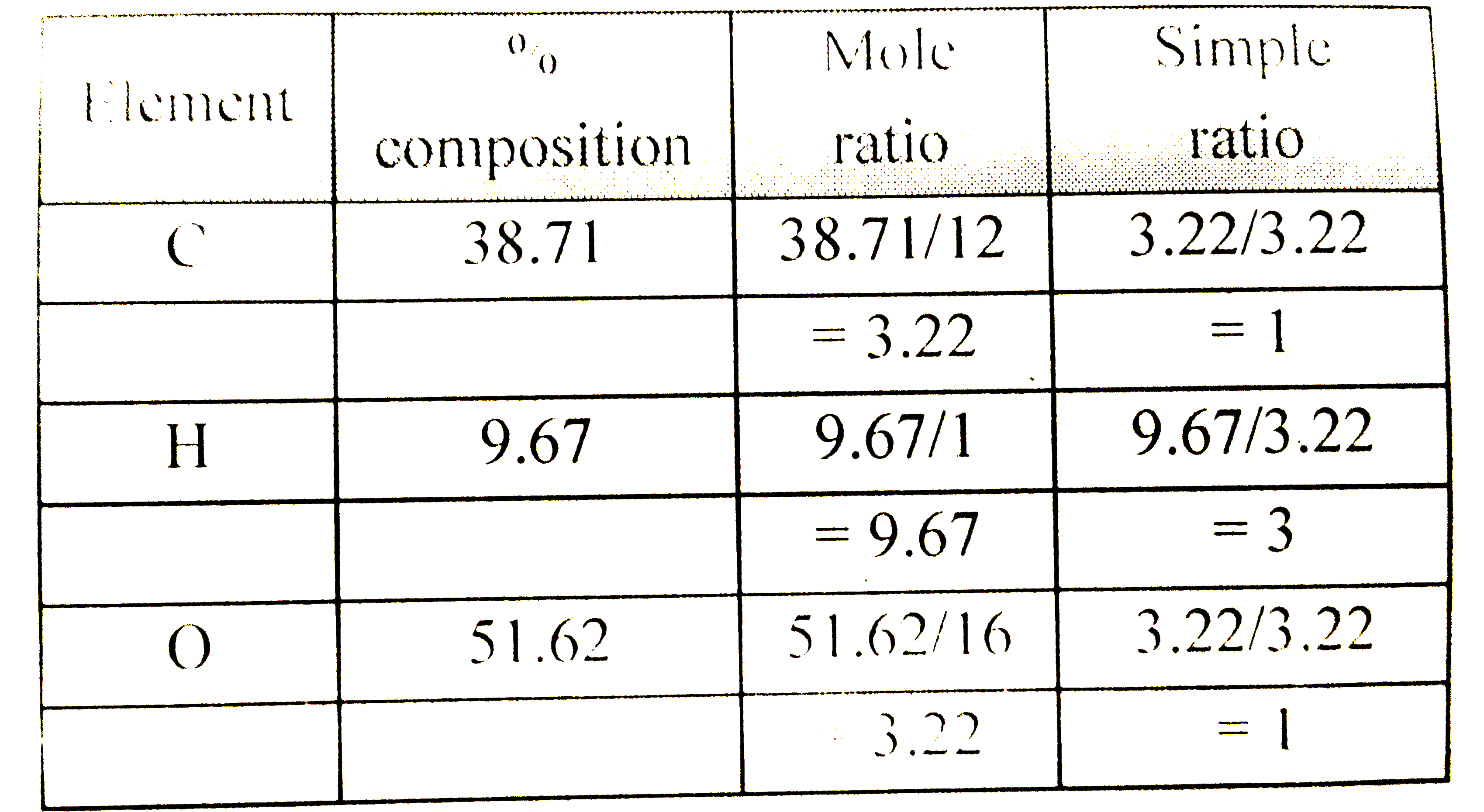
- An organic compound contains carbon, hydrogen and oxygen. Its elementa...
Text Solution
|
- In which of the following coordination entites the magnitude of Delta(...
Text Solution
|
- The alkali metals form salt like hydrides by the direct synthesis at e...
Text Solution
|
- Which of the following are not state functions? (I) q+w (II)q (I...
Text Solution
|
- n the hydrocarbon, overset(6)CH(3)-overset(5)CH=overset(4)CH-overset...
Text Solution
|
- Number of moles of MnO(4)^(-) required to oxidise one mole of ferrous ...
Text Solution
|
- The rate constant k(1) and k(2) for two different reactions are 10^(16...
Text Solution
|
- A strong base can abstract an alpha-hydrogen from
Text Solution
|
- Standard free energies of formation (I kJ //mol ) at 298 K are -237 ....
Text Solution
|
- If 'a' stands for the edge length of the cubic systems: simple cubic,b...
Text Solution
|
- For vaporization of water at 1 atmospheric pressure the values of Delt...
Text Solution
|
- A 0.66 kg ball is moving wih a speed of 100 m//s. The associated wavel...
Text Solution
|
- Three moles of an ideal gas expanded spontaneously into vacuum. The wo...
Text Solution
|
- The following teo reaction are known : Fe(2)O(3)(s)+3CO(g)rarr2Fe(s)...
Text Solution
|
- The reaction, 2A(g) + B(g)hArr3C(g) + D(g) is begun with the conce...
Text Solution
|
- The pressure exerted by 6.0 g of methane gas in a 0.03 m^(3) vessel at...
Text Solution
|
- Which of the following expressions correctly repesents the equivalent ...
Text Solution
|
- How many bridging oxygen atoms are presents in P(4)O(10) ?
Text Solution
|