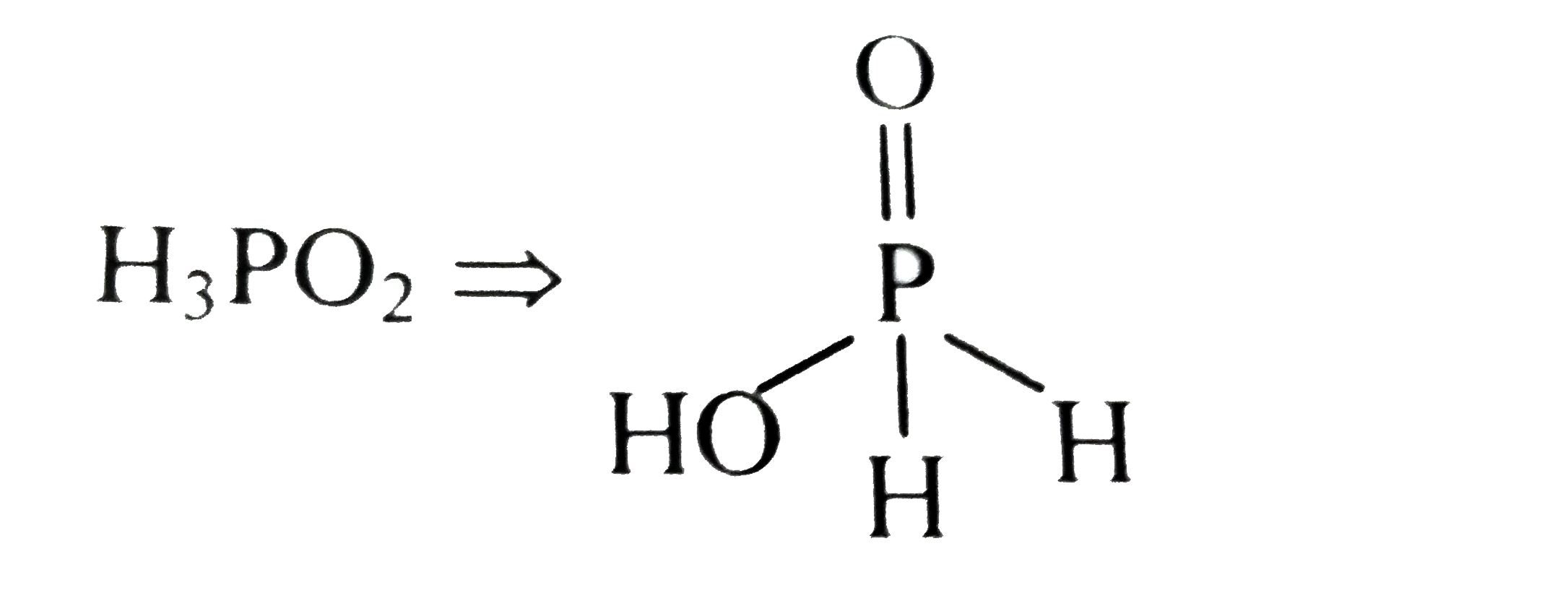
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NEET
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise CHEMISTRY|151 VideosNEET
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise QUESTION|92 VideosMETALLURGICAL OPERATIONS
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise MCQ|21 VideosNEET 2018
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise QUESTION|45 Videos
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-NEET-MCQ
- Strong reducing behaviour of H(3)PO(2) is due to
Text Solution
|
- In a zero-order reaction for every 10^(@) rise of temperature, the rat...
Text Solution
|
- Which of the following pairs is isostractural (i.e having the same sha...
Text Solution
|
- In which of the following reactions,standard reaction entropy change(D...
Text Solution
|
- In a reaction , A + B rarr Product, rate is doubled when the concentra...
Text Solution
|
- Limiting molar conductivity of NH(4)OH [i.e., Lambda(m)^(@)(NH(4)OH)] ...
Text Solution
|
- Which of the following species contains three bond pair and one lone ...
Text Solution
|
- Buffer solutions have constant acidity and alkalinity because
Text Solution
|
- In freundlich adsorption isotherm, the value of 1/n is :
Text Solution
|
- pH of saturated solution of Ba(OH)(2) is 12. The value of solubility p...
Text Solution
|
- When Cl(2) gas reacts with hot and concentrated sodium hydroxide solut...
Text Solution
|
- Which one of the following statements is incorrect about enzyme catal...
Text Solution
|
- P(A)and P(B) are the vapour pressure of pure liquid components ,Aand B...
Text Solution
|
- The protcting power of lyophilic colloidal solution is expressed in te...
Text Solution
|
- Maximum number of electrons in a sub-shell with l = 3 and n = 4 is.
Text Solution
|
- 50 mL of each gas A and of gas B takes 150 and 200 seconds respectivel...
Text Solution
|
- Standard enthalpy of vaporisationDeltaV(vap).H^(Theta) for water at 10...
Text Solution
|
- The total number of octahedral void (s) per atom present in a cubic cl...
Text Solution
|
- Correct set of four quantum numbers for the valence (outermost) electr...
Text Solution
|
- a metal crystallizes with a face-centered cubic lattice.The edge of th...
Text Solution
|
- The enthalpy of fusion of water is 1.435 kcal//"mole". The molar entro...
Text Solution
|