Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
PRADEEP|Exercise Curiosity Question|2 VideosSOLID STATE
PRADEEP|Exercise Problem For Pactice|6 VideosSOLID STATE
PRADEEP|Exercise Advanced problems|19 VideosS-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS)
PRADEEP|Exercise Assertion -Reason Type Question (Type 2)|19 VideosSOME p-BLOCK ELEMENTS
PRADEEP|Exercise Competition Focus (JEE( Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions (Type I)|23 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-SOLID STATE -Sample problem
- Br^- ions form a close packed structure. If the radius of Br^– ions is...
Text Solution
|
- Xenon crystallizes in the face-centred cubic lattice and the edge of t...
Text Solution
|
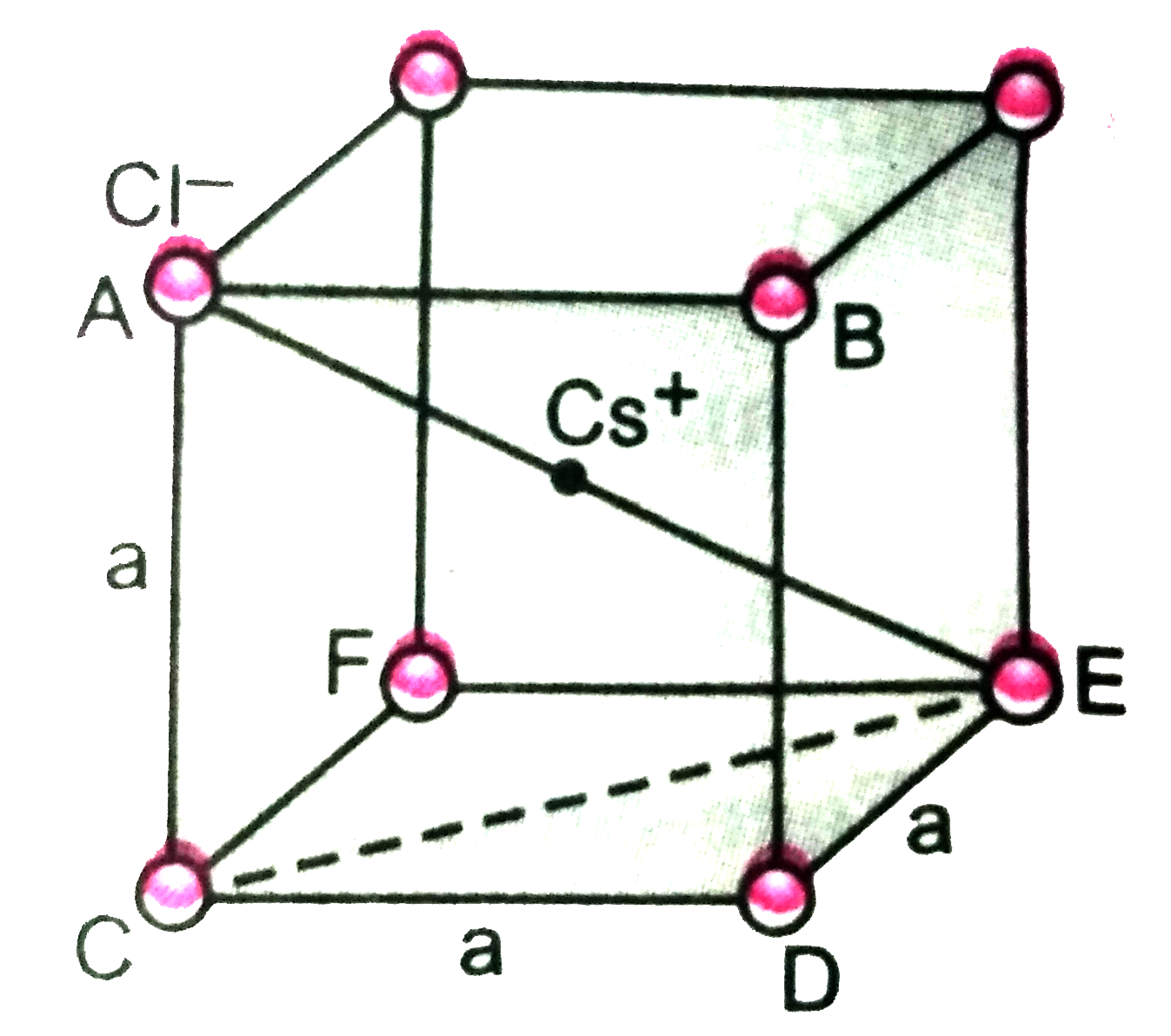
- CsCl has bc c arrangement and its unit cell edge length is 400 pm. Cal...
Text Solution
|
- Sodium metal crystallises in body centred cubic lattic with the cell e...
Text Solution
|
- In face - centred cubic (fcc) crystal lattice, edge length is 400 pm....
Text Solution
|
- Silver froms ccp lattice and X-ray studies of its crystals show that t...
Text Solution
|
- Sodium has a bc c structure with nearest neighbour distance of 365.9 p...
Text Solution
|
- Gold (atomic mass = 197 u) has atomic radius = 0.144 nm. It crystalli...
Text Solution
|
- Gold has a close-packed structure which can be viewed as-spheres occup...
Text Solution
|
- CsCl has cubic structure. Its density is 3.99 g cm^(-3). What is the d...
Text Solution
|
- The density of aluminium is 2700 " kg m^(-3) , Aluminium crytallise...
Text Solution
|
- The edge length of unit cell of a metal having molecular weight 75 g/m...
Text Solution
|
- Calculate the value of Avogadro's number from the following data: De...
Text Solution
|
- The density of KCl is 1.9893g cm^(-3) and the length of a side unit ce...
Text Solution
|
- X-rays diffraction studies show that copper crystallizes in an fcc uni...
Text Solution
|
- An element crystallizes into a structure which may be describes by a c...
Text Solution
|
- Density of Li is 0.53"g cm"^(-3). The edge length of Li is 3.5Å. Find ...
Text Solution
|
- The density of KBr is 2.75 " g cm^(-3) , The length of edge of the un...
Text Solution
|
- The density of copper metal is 8.95" g "cm^(-3). If the redius of copp...
Text Solution
|
- If NaCl is doped with 10^(-3) mol percent of SrCI(2), what is the conc...
Text Solution
|