A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-APPENDIX-MODEL TEST PAPER <br> (Section C )
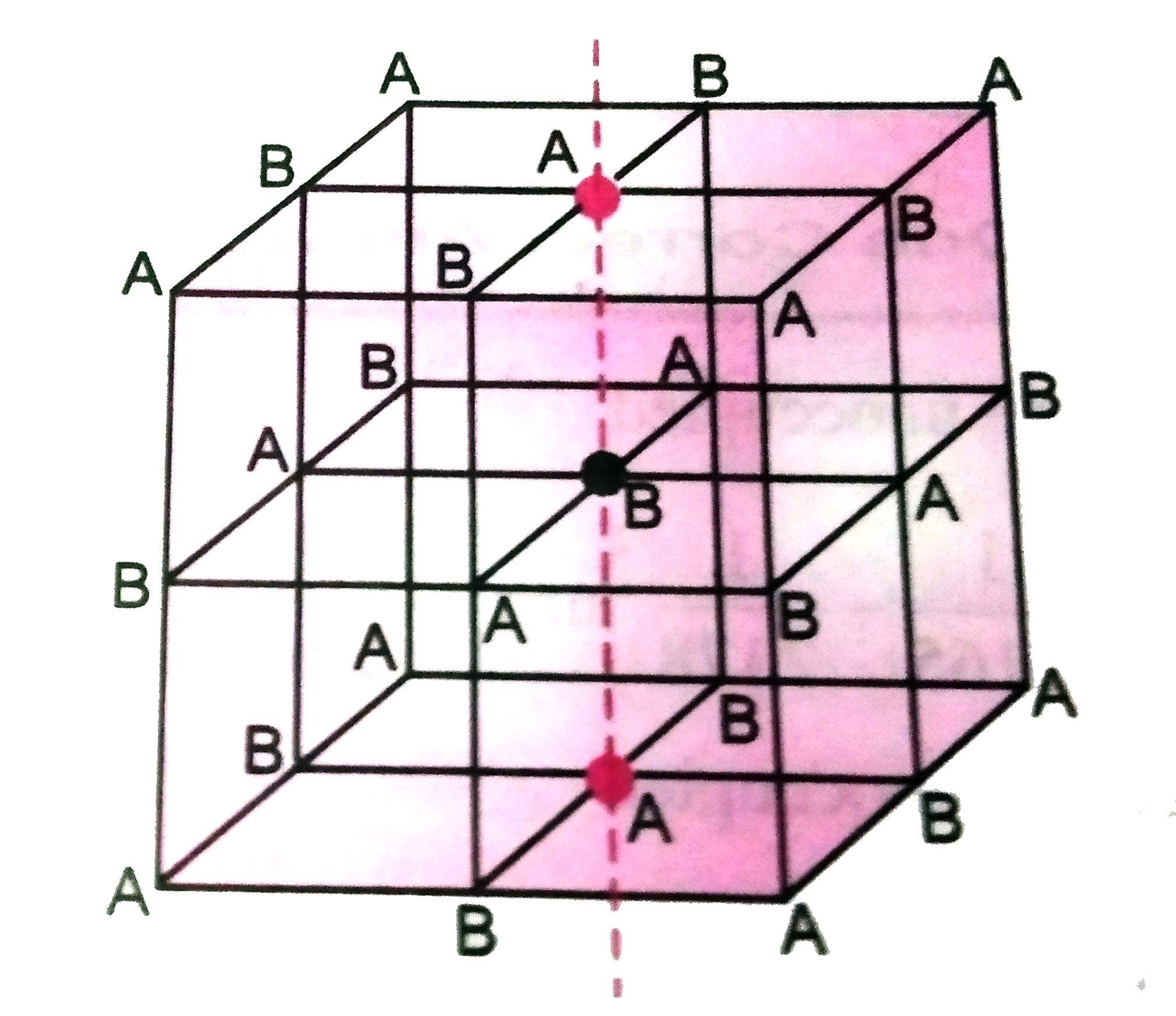
- In a solid AB having the NaCl structure, A atom occupies the corners o...
Text Solution
|
- Calcium carbonate reacts with aqueous HCl to give CaCl(2) and CO(2) ac...
Text Solution
|
- Arrange the following in the order of property indicated for each set:...
Text Solution
|
- (a) Which gases are responsible for greenhouse effect ? List som...
Text Solution
|
- Naturally occurring boron consists of two isotopes whese atomic we...
Text Solution
|
- Calculate the energy of C-Cl bond from the following data : CH(4)(g...
Text Solution
|
- Calculate the wave number for the longest wavelength transition...
Text Solution
|
- Two vessels of capactie 1.5 L and 2.0 L containing hydrogen at 750 mm ...
Text Solution
|
- (i) An element of group 2 form covalent oxide which is amphoteric...
Text Solution
|
- (i) Complete combustion of the hydrocarbon gives 0.66 g of CO(2)...
Text Solution
|