Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
PRADEEP|Exercise Problem|35 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Curiosity Question|6 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (ASSERTION-REASON)|17 VideosTHERMODYNAMICS
PRADEEP|Exercise MULTIPLE CHOICE QUESTION ( BASED ON PRACTICAL CHEMISTRY)|3 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STRUCTURE OF ATOM-Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))
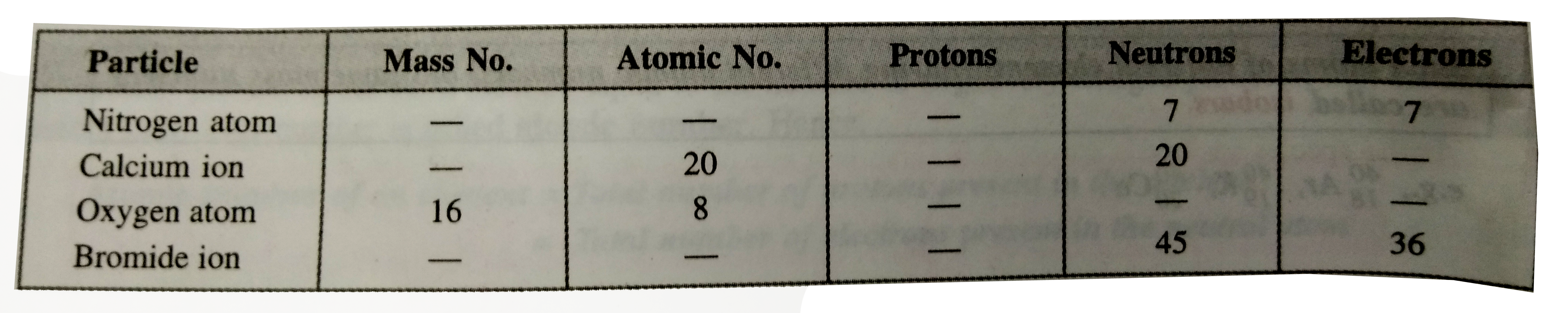
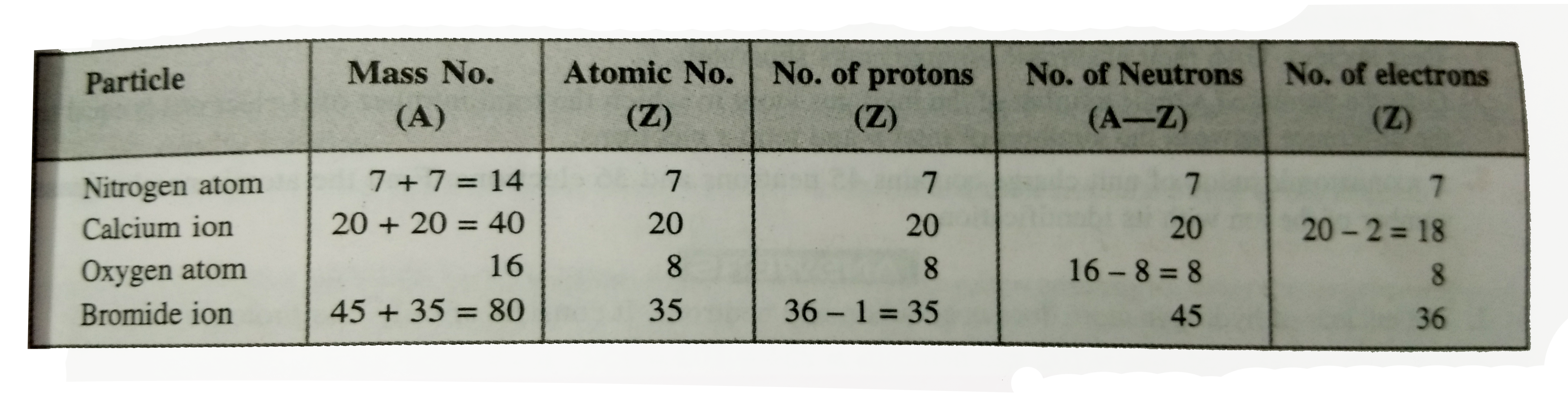
- Complete the following tables:
Text Solution
|
- Assertion. Cathode rays originate from the cathode and move towards an...
Text Solution
|
- Assertion. According to Thomson model of atom, mass of the atom is con...
Text Solution
|
- Assertion(A): All isotopes of a given element show the same type of ch...
Text Solution
|
- Assertion (A) : Hydrogen has only one electron in its 1s orbital but i...
Text Solution
|
- Assertion. In Lyman series of H-spectra, the maximum wavelength of lin...
Text Solution
|
- Assertion. The plots of probability density and radial probability fun...
Text Solution
|
- Assertion (A) : A spectral line will be seen for 2p(x)-2p(y) transitio...
Text Solution
|
- Assertion : The energy of an electron is mainly determined by principa...
Text Solution
|
- Assertion. Electronic configurations of Cr^(3+) (containing 21 electro...
Text Solution
|
- STATEMENT-1 : The 19^(th) electron in potassium atom enters into 4s-or...
Text Solution
|
- Assertion. Only principal quantum number determines the energy of an e...
Text Solution
|
- The free gaseous Cr atom has six unpaired electrons. Half-filled s-o...
Text Solution
|